Vmp Template
Vmp Template - Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web (validation master plan) or lower tier documentation alone may cover the qualification of materials. Web preview validation master plan template. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. In these circumstances it is only the bold and confident that. Web validation master plans discuss validation activities across an entire site or within an organization. Web what is validation master plan (vmp): Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. Tell them what you’re going to do (vmp). Web validation master plans discuss validation activities across an entire site or within an organization. Documents include placeholder marks for all. Web the validation master plan considering the above, we can now complete the validation program adage: Web preview validation master plan template. Tell them what you’re going to do (vmp). Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. Web validation master plans discuss validation activities. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. Web (validation master plan) or lower tier documentation alone may cover the qualification of materials. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor. Web what is validation master plan (vmp): The validation master plan is a summary of validation strategy. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. In these circumstances it is only the bold and confident that. Web validation master plan. Web validation templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. The document is fully editable so that you can adapt it to your company design. Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. Web what is validation. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. Tell them what you’re going to do (vmp). The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web validation master. Web preview validation master plan template. Web the purpose of this validation master plan (hereinafter known as the “plan” or “vmp”) is to provide guidelines and protocol for the validation of applicable processes, equipment,. Web in this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and. Web validation master plan individual validation plan manufacturers often choose to develop a validation master plan (vmp) as a tool to control and monitor the status of validation. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. In these circumstances it is only the bold and confident that.. 5.2.8 the headings of the vmp may be based on the above list. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Web master validation. The objective of this document is to outline the validation plan for a gmp site and to ensure that all the. The validation master plan is a summary of validation strategy. Web the validation master plan considering the above, we can now complete the validation program adage: Web master validation plan is a strategic document which identifies the elements to. Web the validation master plan considering the above, we can now complete the validation program adage: Web in this comprehensive guide, we’ll address key questions such as “what does a validation master plan include?” and “how can i create a vmp?” and “who is responsible for. The document is fully editable so that you can adapt it to your company design. Web preview validation master plan template. The validation master plan serves as a roadmap that helps to set the course, justifying the strategy, outlined the test and. Documents include placeholder marks for all. Web a validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a pharmaceutical or medical device. Web validation master plans discuss validation activities across an entire site or within an organization. 5.2.8 the headings of the vmp may be based on the above list. Web validation templates design is of critical importance when regulatory compliance and clarity of purpose are prerequisites. Web a validation master plan (vmp), a segment of gmps (good manufacturing practices) for pharmaceutical, biotech and medical device organizations, is a report that. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. Web validation master plan template document control details this will include details such as vmp reference number, version number, date and authorisation signatures. Tell them what you’re going to do (vmp). Web the purpose of this validation master plan (hereinafter known as the “plan” or “vmp”) is to provide guidelines and protocol for the validation of applicable processes, equipment,. Web master validation plan is a strategic document which identifies the elements to be validated, the approach to be taken for validation of each element, the.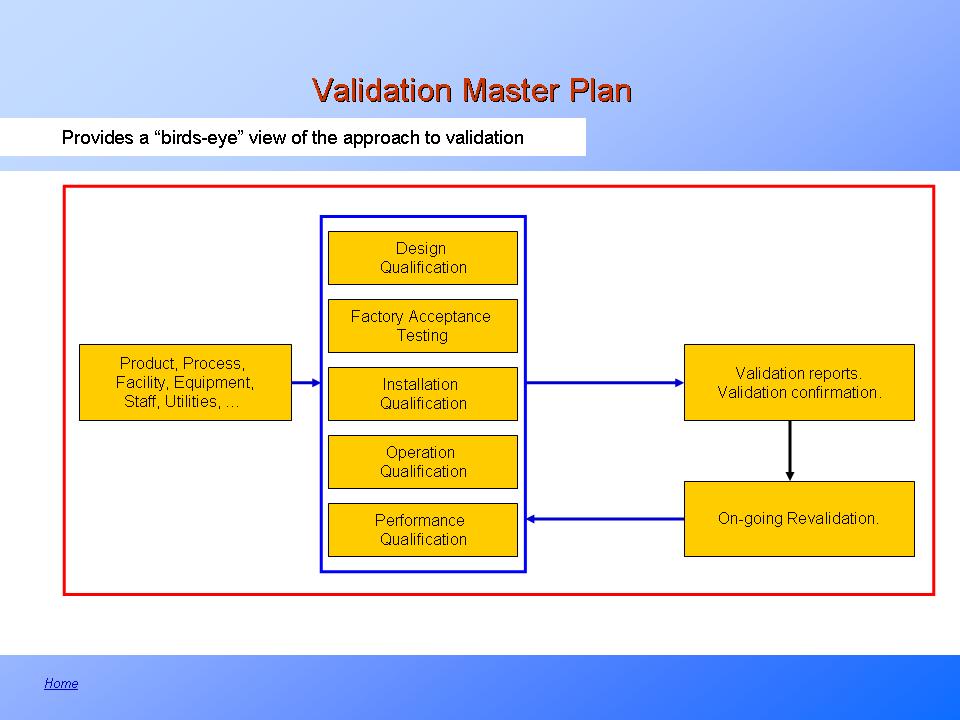
Overview of the Validation Master Plan PresentationEZE

Validation Master Plan. Understand the importance and benefits

Validation Master Plan Bio Chem Shop
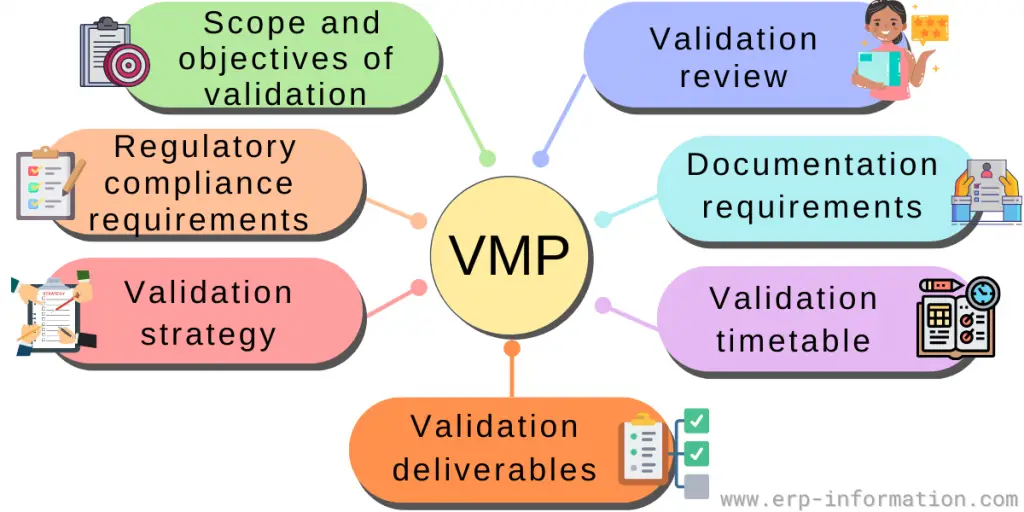
What is Validation Master Plan? (Template, Examples)

10+ Validation Plan Templates Sample Templates
VMP Template PDF Verification And Validation Business Process

Validation Master Plan (VMP) Template LexDocPharma

How to create a Validation Master Plan in 5 steps. Templates & more
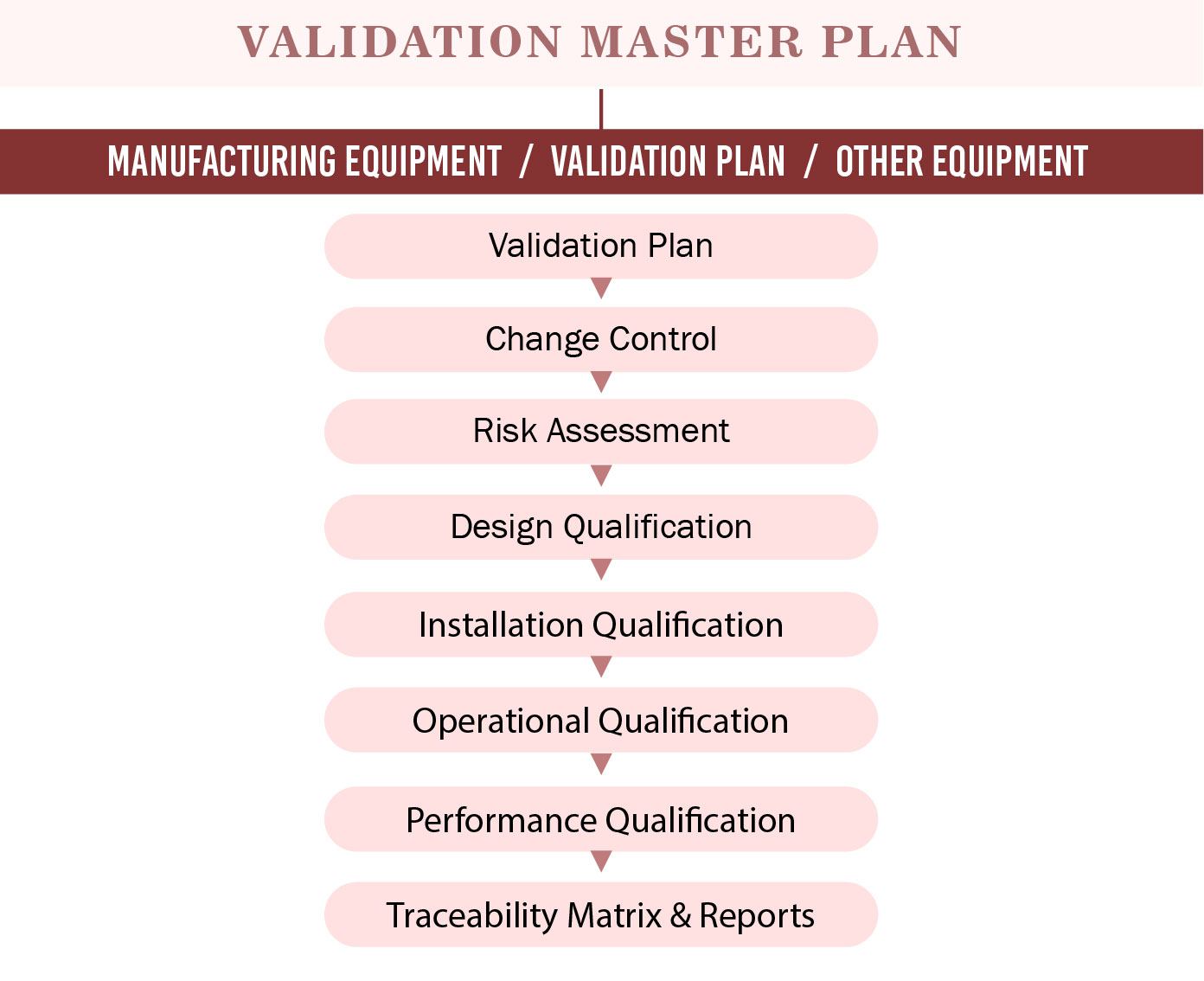
Medical Device Validation Master Plan (VMP) Consultancy Firm Operon
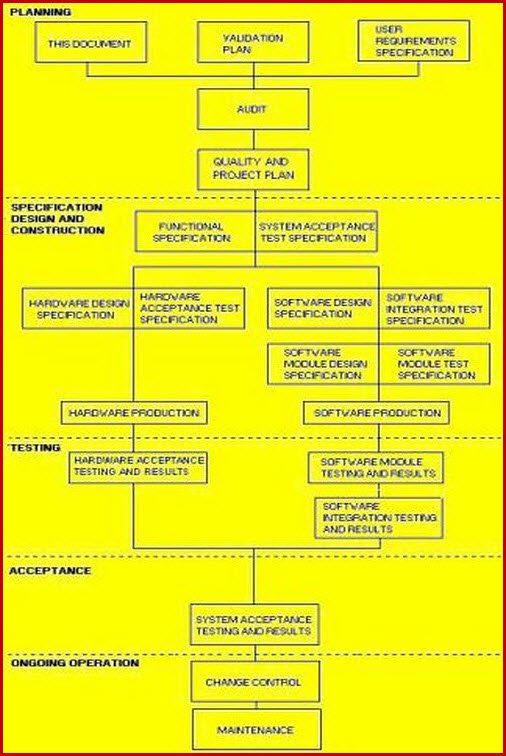
Validation Master Plan (VMP) Downloadable Interactive Template.
The Objective Of This Document Is To Outline The Validation Plan For A Gmp Site And To Ensure That All The.
The Validation Master Plan Is A Summary Of Validation Strategy.
Web Validation Master Plan Individual Validation Plan Manufacturers Often Choose To Develop A Validation Master Plan (Vmp) As A Tool To Control And Monitor The Status Of Validation.
In These Circumstances It Is Only The Bold And Confident That.
Related Post:
