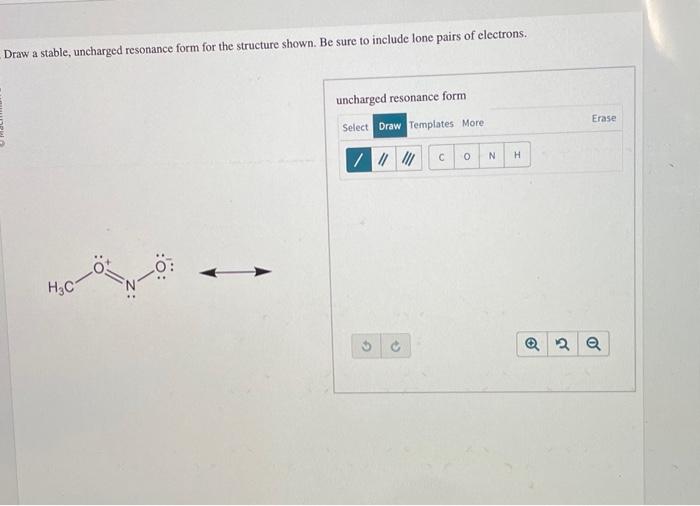
Draw The Lewis Structure Of Hcn Include Lone Pairs
Draw The Lewis Structure Of Hcn Include Lone Pairs - Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Put one electron pair in each bond 4. Web moving one lone pair from each terminal o atom, the following structure is obtained. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure of. By signing up, you accept quizlet's terms of service and privacy policy. Add lone pairs around n and c to satisfy the octet rule. 3 lone pairs (6 electrons) on each f. Using lewis dot symbols to describe covalent bonding. Web to draw the lewis structure of hcn, place h at the center and connect it to c. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. This sharing of electrons allowing atoms to stick together is the basis of covalent bonding. Web the lewis structure indicates that each cl atom has three pairs of electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Create an account to view solutions. Step 1/6**step 1:** count the total number of valence electrons in hcn. Each atom should have eight electrons around them because. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not. Web first of all, to remind you lewis’s structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound. Draw the lewis structure of. Step 1/6**step 1:** count the total number of valence electrons in hcn. Draw the lewis structure of hcn.hcn. You'll get a detailed solution from a subject. Fill outer atoms with electrons 5. Each atom should have eight electrons around them because. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Put one electron pair in each bond 4. Web moving one lone pair from each terminal o atom, the following structure is obtained. Web the lewis structure indicates that. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Each atom should have eight electrons around them because. Web first of all, to remind you lewis’s structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound. Add lone pairs around n and. Create an account to view solutions. Step 1/6**step 1:** count the total number of valence electrons in hcn. This sharing of electrons allowing atoms to stick together is the basis of covalent bonding. This is the complete lewis structure of co 2. 2 single bonds between the f and o. Each atom should have eight electrons around them because. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Web this problem has been solved! Create an account to view solutions. Put least electronegative atom in centre 3. Add lone pairs around n and c to satisfy the octet rule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Step 1/6**step 1:** count the total number of valence electrons in hcn. 3 lone pairs (6 electrons) on each f. Web for the hcn lewis structure, calculate the total number of valence. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. By signing up, you accept quizlet's terms of service and privacy policy. Add lone pairs around n and c to satisfy the octet rule. Web to draw the lewis structure of hcn, place h at the center and connect it to c. Web. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Add lone pairs around n and c to satisfy the octet rule. Web to draw the lewis structure of hcn, place h at the center and connect it to c. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. 3 lone pairs (6 electrons) on each f. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Draw the lewis structure of hcn.hcn. Using lewis dot symbols to describe covalent bonding. Put one electron pair in each bond 4. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Web first of all, to remind you lewis’s structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound. Web for the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Step 1/6**step 1:** count the total number of valence electrons in hcn. 2 single bonds between the f and o. Web moving one lone pair from each terminal o atom, the following structure is obtained. Web this problem has been solved!
HCN Lewis Structure (Hydrogen Cyanide) YouTube
Solved Draw the Lewis structure of HCN. Include lone pairs.

Estrutura De Lewis Hcn
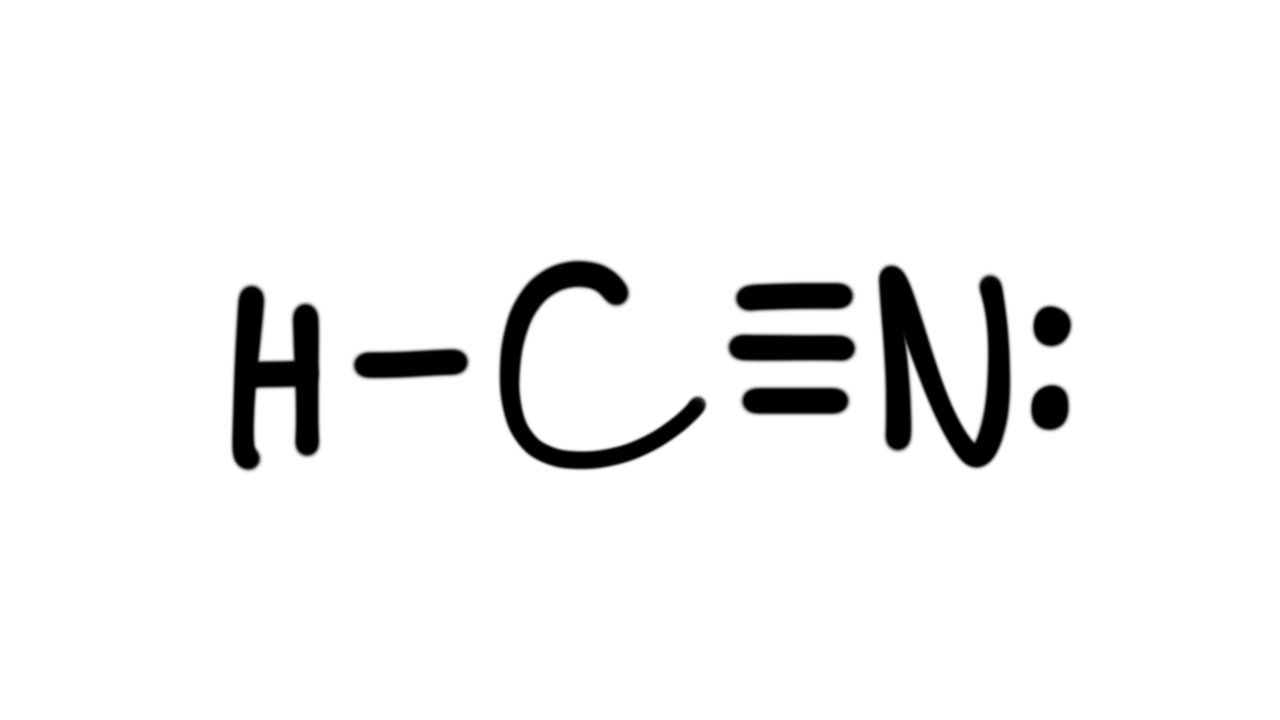
Estrutura De Lewis Hcn
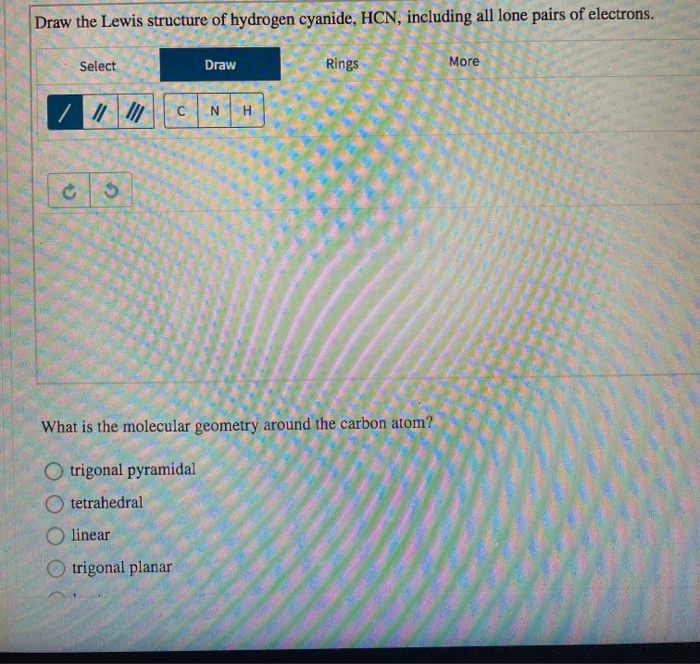
Draw The Lewis Structure Of Hydrogen Cyanide, HCN, Including All Lone
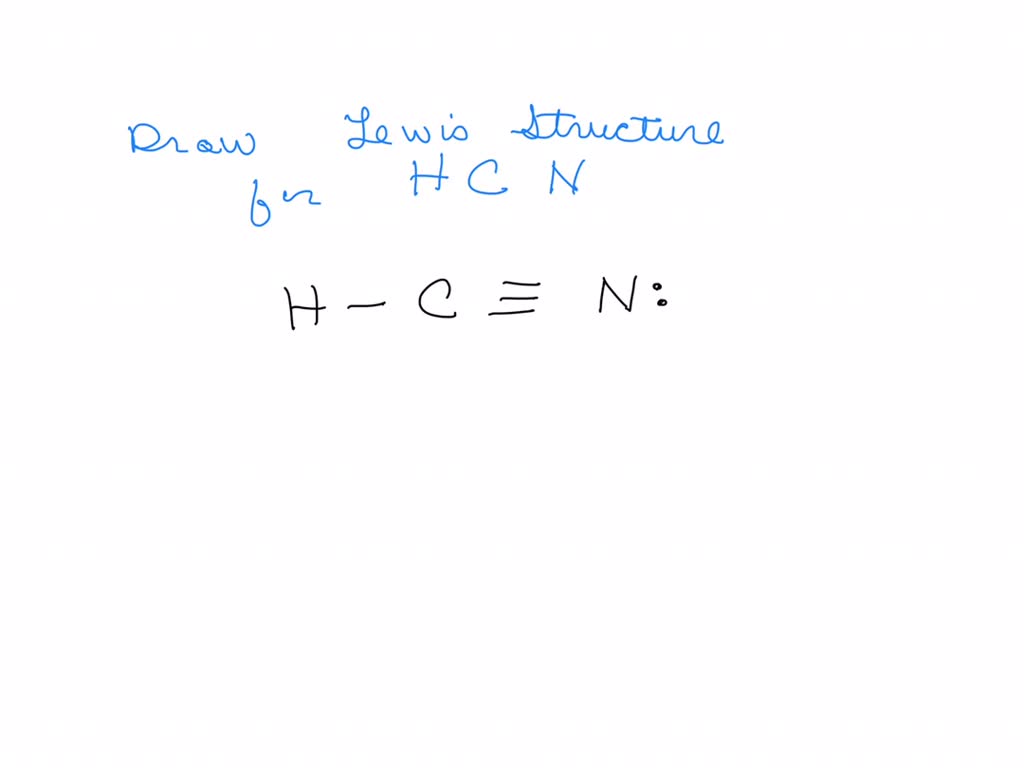
SOLVED Draw the Lewis structure of HCN. Include lone pairs

Lewis Dot Diagram Of Hcn
[Solved] Draw the Lewis structure of HCN. Include lone pairs. Select
How To Draw The Lewis Structure Of Hcn Including Lone Pairs
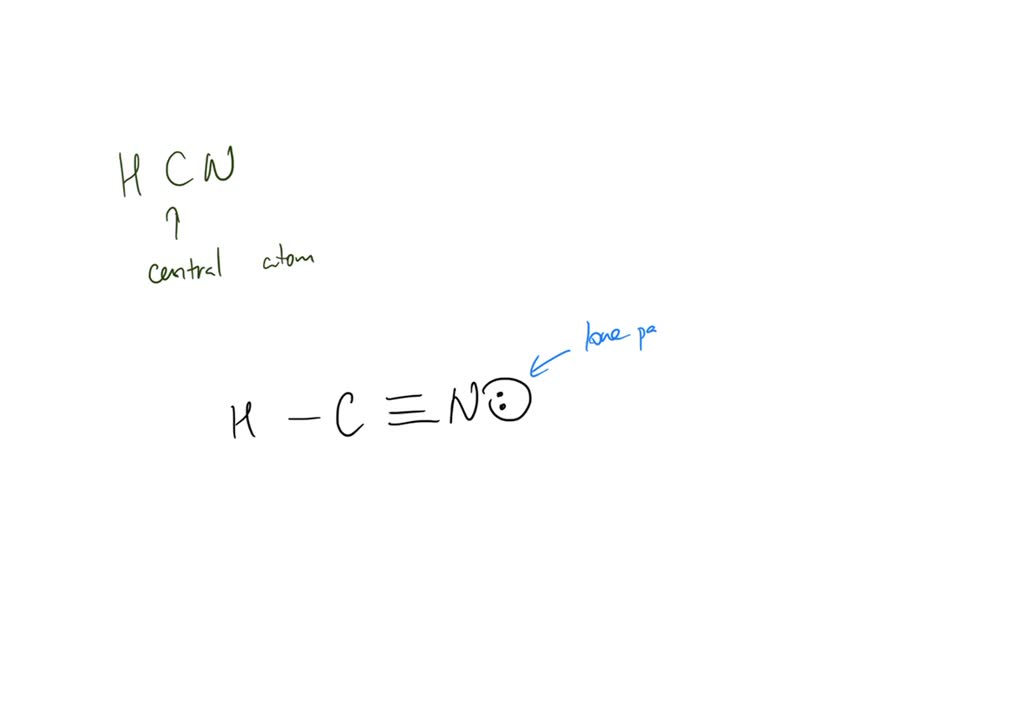
SOLVED Draw the Lewis structure of HCN. Include lone pairs; Select
Create An Account To View Solutions.
Put Least Electronegative Atom In Centre 3.
Fill Outer Atoms With Electrons 5.
This Is The Complete Lewis Structure Of Co 2.
Related Post: