What Electron Pattern Can Be Observed With The Noble Gases
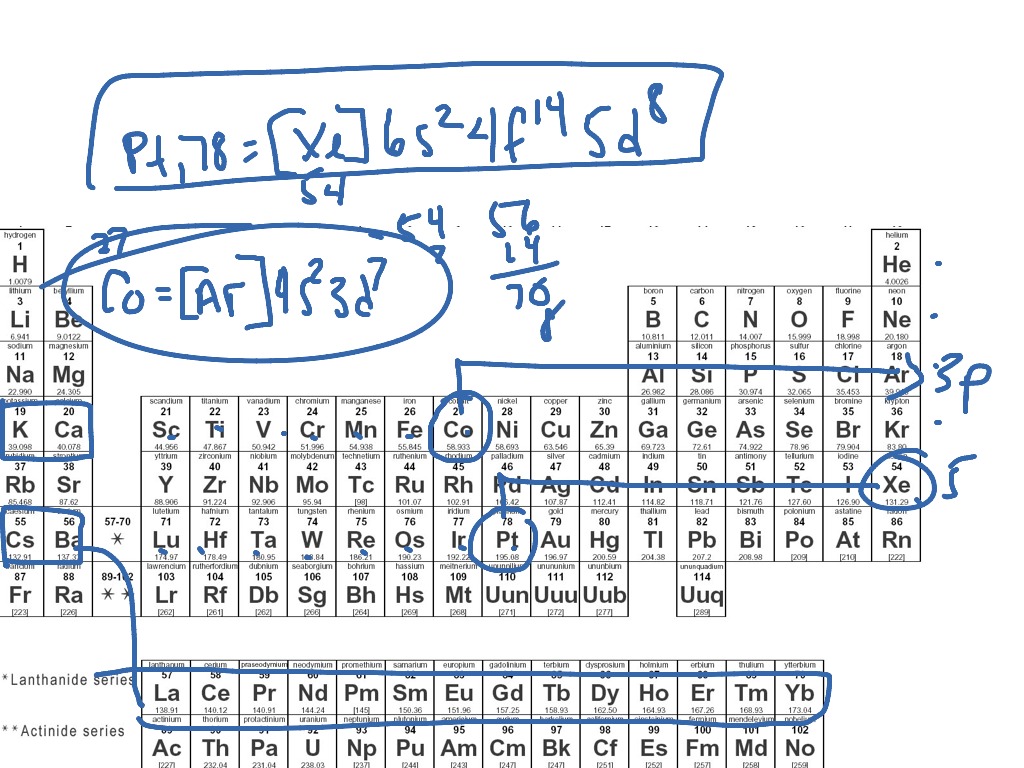
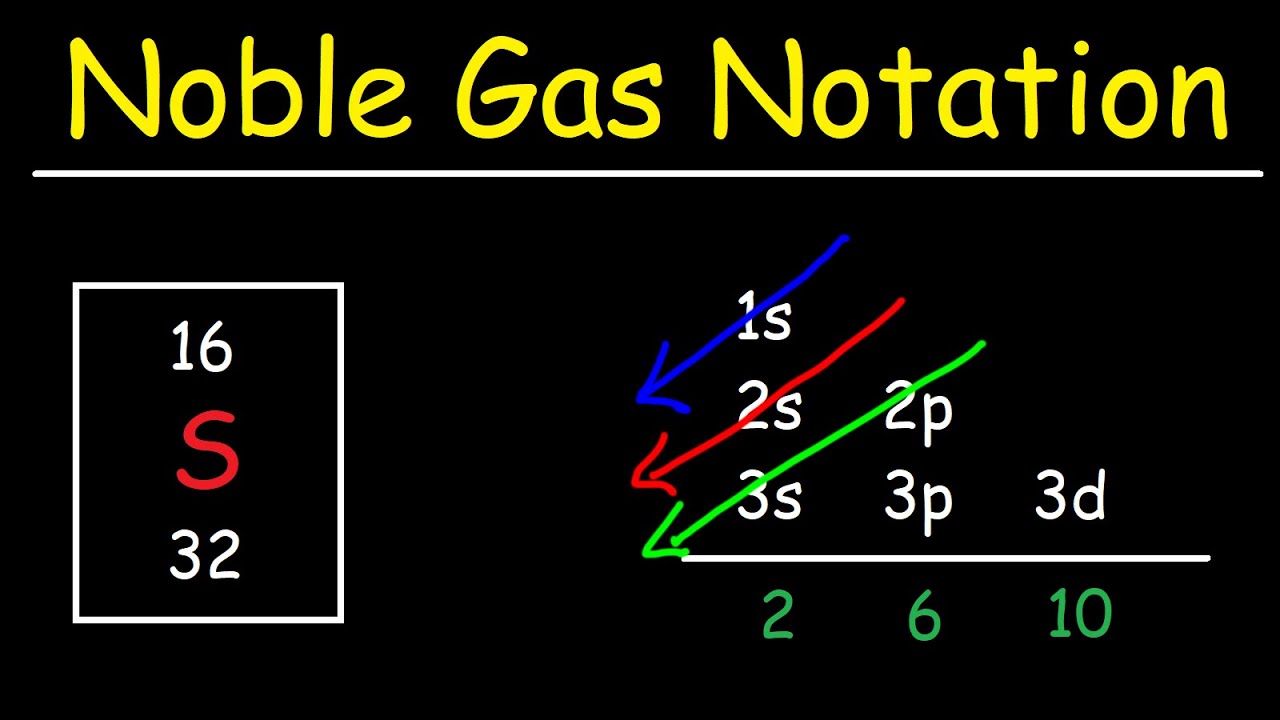
What Electron Pattern Can Be Observed With The Noble Gases - Then the remaining electrons are listed explicitly. Scientists could have picked any group of elements, but the noble gases prevail because of their completely filled shells and stability. This full electron configuration makes them very stable and inert or nonreactive. Web the noble gases, in the column on the right, almost never react, since they have eight valence electrons, which makes it very stable. The noble gases are present in the last group of the periodic table having the maximum possible number of electrons allowed for that period in which they are. Web there are six noble gases and have the following electronic configuration. Web the electron configuration and the orbital diagram are: Web what electron pattern can be observed with the noble gases? The helium atom contains two protons and two electrons. For helium, that limit is just two electrons. It was observed that this radioactive gas is present in very small amounts in soils and minerals. The other gases have full outer shells of two \(s\) and six \(p\) electrons. Then the remaining electrons are listed explicitly. Only highly electronegative elements can form stable compounds with the noble gases in positive oxidation states without being oxidized themselves. The elements. Noble gases are found at the end of the periodic table (group 18) and consist of the maximum number of electrons allowed for that particular period. Web it was observed that highly recognizable unique micro plasma pattern formations can be controlled on a large scale by varying the discharge key parameters and driving modes. This full electron configuration makes them. The noble gases are present in the last group of the periodic table having the maximum possible number of electrons allowed for that period in which they are. Only highly electronegative elements can form stable compounds with the noble gases in positive oxidation states without being oxidized themselves. Would it be [ar] or [ne]3s^23p^6? Web potassium has nineteen electrons, one. For helium, that limit is just two electrons. Only highly electronegative elements can form stable compounds with the noble gases in positive oxidation states without being oxidized themselves. The ionization energies of the noble gases decrease with increasing atomic number. Each f shell holds up to 14 electrons. The ionization potential decreases with an increasing radius, because the valence electrons. Would it be [ar] or [ne]3s^23p^6? This led chemists to think of them as totally unreactive. It was observed that this radioactive gas is present in very small amounts in soils and minerals. Web on the right hand column of the periodic table, you will see elements in group 0. Web potassium has nineteen electrons, one more than the noble. Following hydrogen is the noble gas helium, which has an atomic number of 2. The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. He = 1s^2 ne =[he]2s^2 2p^6 ar = [ne]3s^2 3p^6 kr = [ar]4s^2 3d^10 4p^6 xe =[kr]5s^2 4d^10 5p^6 rn =[xe]6s^2 4f^14 5d^10 6p^6 the electron pattern is that all of them have fully. The other gases have full outer shells of two \(s\) and six \(p\) electrons. Web it was observed that highly recognizable unique micro plasma pattern formations can be controlled on a large scale by varying the discharge key parameters and driving modes. Then the remaining electrons are listed explicitly. Web the noble gases, in the column on the right, almost. Web the electron configuration and the orbital diagram are: The helium atom contains two protons and two electrons. Web what would the noble gas electron configuration for an element look like if the element is a noble gas? Helium has a full outer shell of two \(s\) electrons. (electron configurations) 3.5 periodic variations in element. Noble gases are found at the end of the periodic table (group 18) and consist of the maximum number of electrons allowed for that particular period. The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. Web noble gases have a full outer electron shell, with either 2 or 8 valence electrons. Web it's just common convention to. The elements considered noble gasses are: The helium atom contains two protons and two electrons. He = 1s^2 ne =[he]2s^2 2p^6 ar = [ne]3s^2 3p^6 kr = [ar]4s^2 3d^10 4p^6 xe =[kr]5s^2 4d^10 5p^6 rn =[xe]6s^2 4f^14 5d^10 6p^6 the electron pattern is that all of them have fully filled subshells. They are therefore held less tightly by the atom.. Web what would the noble gas electron configuration for an element look like if the element is a noble gas? The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2). Web the noble gases are in group viii of the periodic table. This led chemists to think of them as totally unreactive. Web noble gases have a full outer electron shell, with either 2 or 8 valence electrons. Web electron configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. Web the elements in group 18 are the noble gases (helium, neon, argon, krypton, xenon, and radon). Following hydrogen is the noble gas helium, which has an atomic number of 2. Scientists could have picked any group of elements, but the noble gases prevail because of their completely filled shells and stability. For example, helium (he) has 2, and neon (ne) has 8 valence electrons. Would it be [ar] or [ne]3s^23p^6? It was observed that this radioactive gas is present in very small amounts in soils and minerals. Helium (he) neon (ne) argon (ar) krypton (kr) xenon (xe) radon (rn) Follow the aufbau rule and write the full electron configuration. Web it's just common convention to reduce the size of the electronic configurations. Web the size of the atom is positively correlated to several properties of noble gases.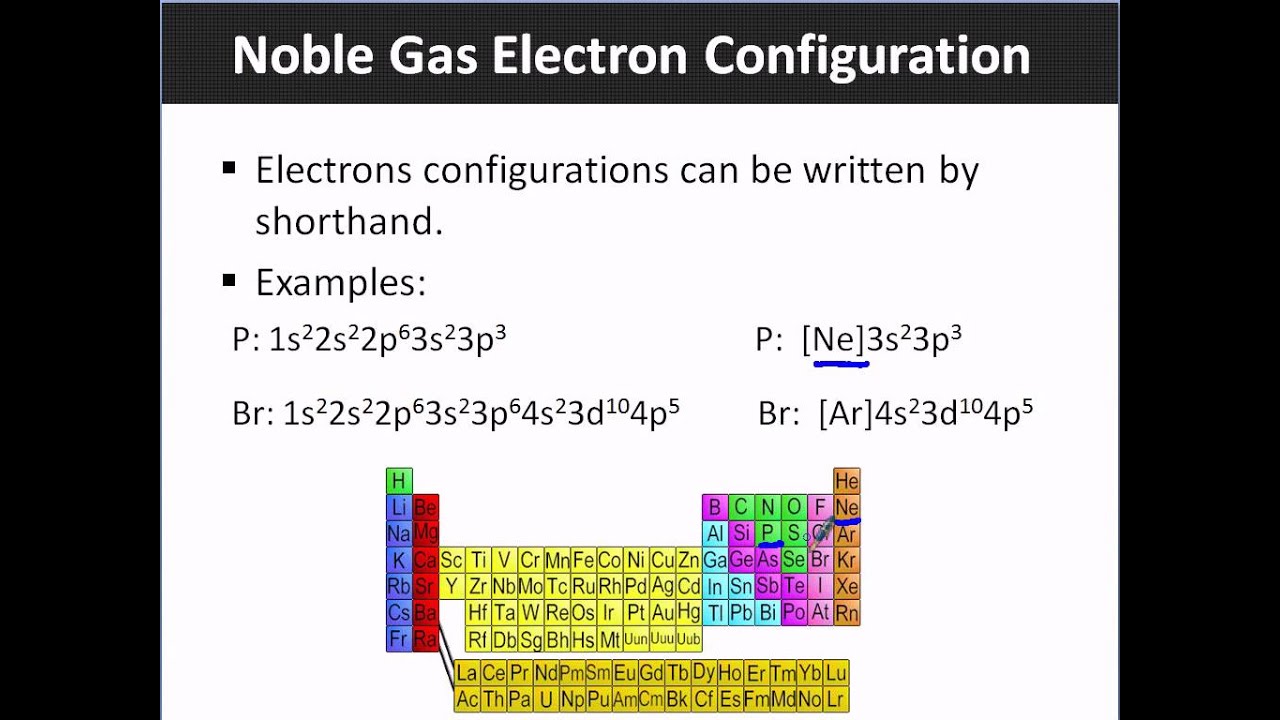
Noble Gas Electron Configurations YouTube
What's So Noble About Noble Gases? Owlcation Education

Electrons in Atoms Lesson7 Noble Gas Configuration YouTube

Electron Configurations with Noble Gas Notation YouTube
Noble Gases Electron Configuration

Noble Gas Electron Configuration Chart
Electron Configuration With Noble Gas Notation YouTube
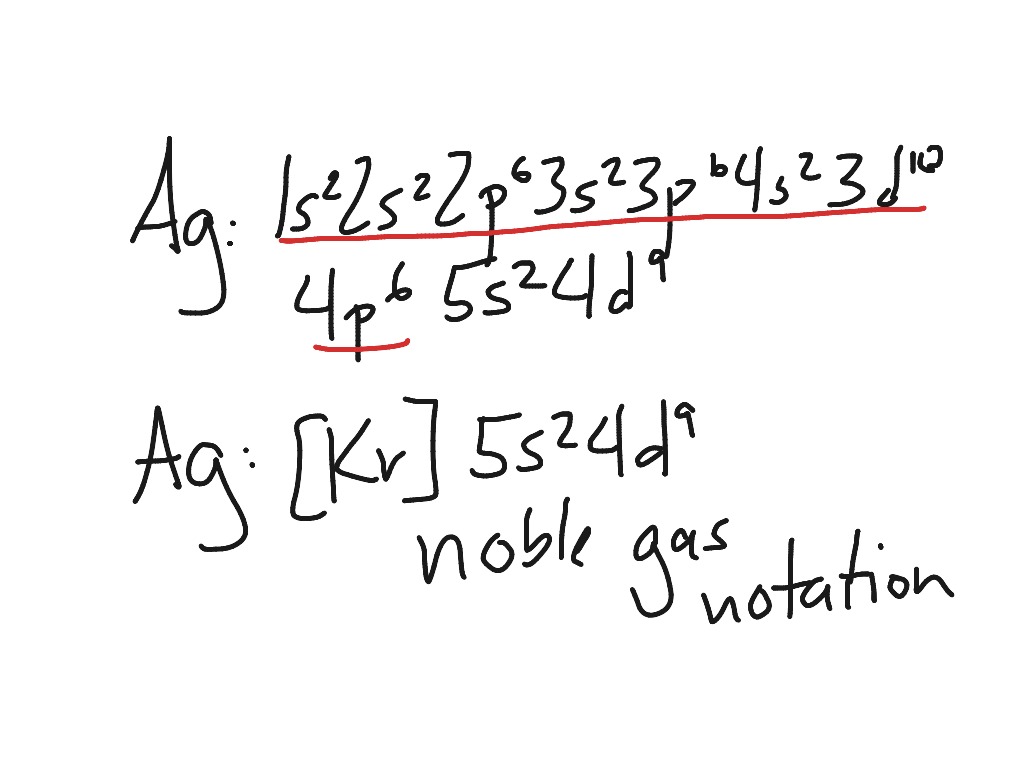
Noble Gases Electron Configuration
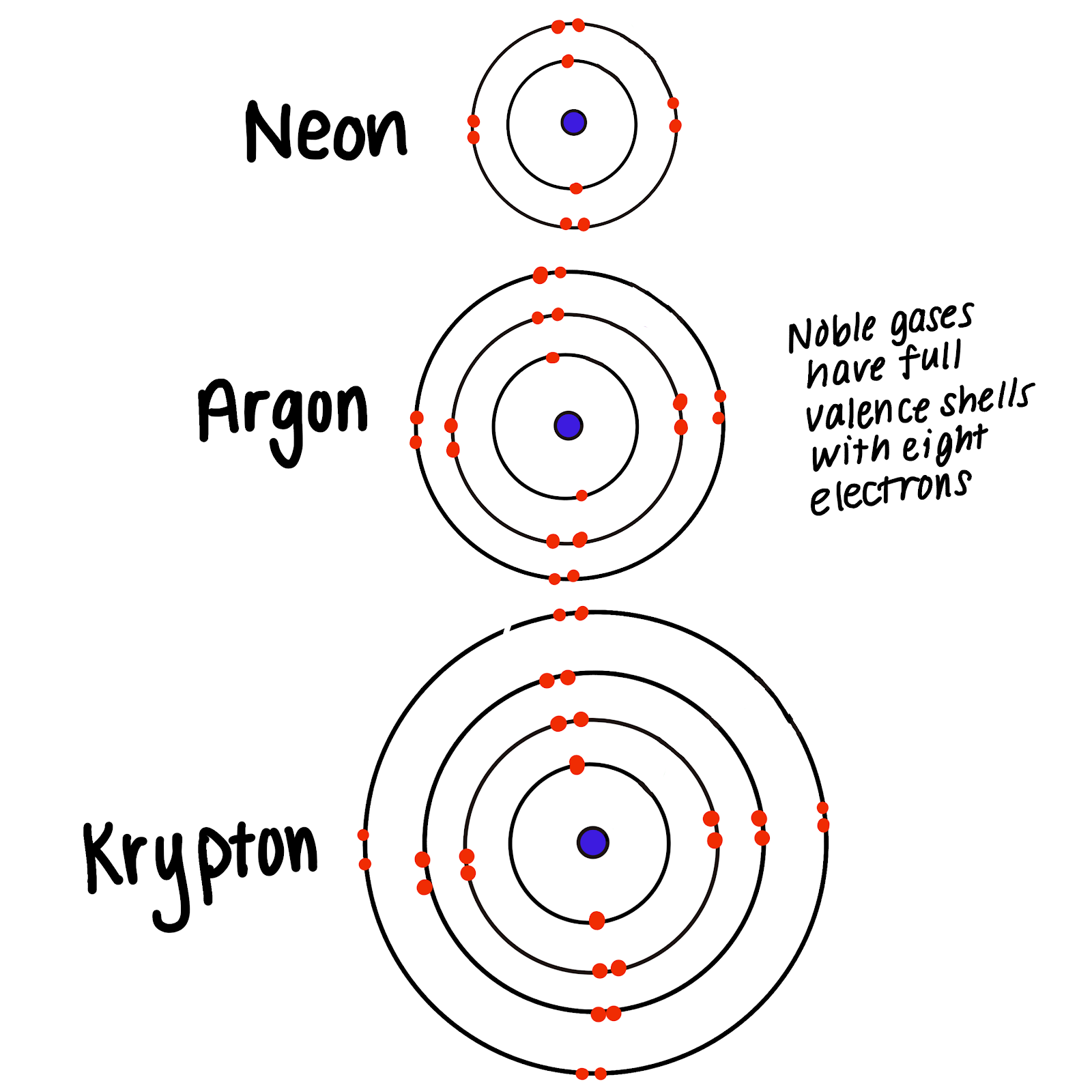
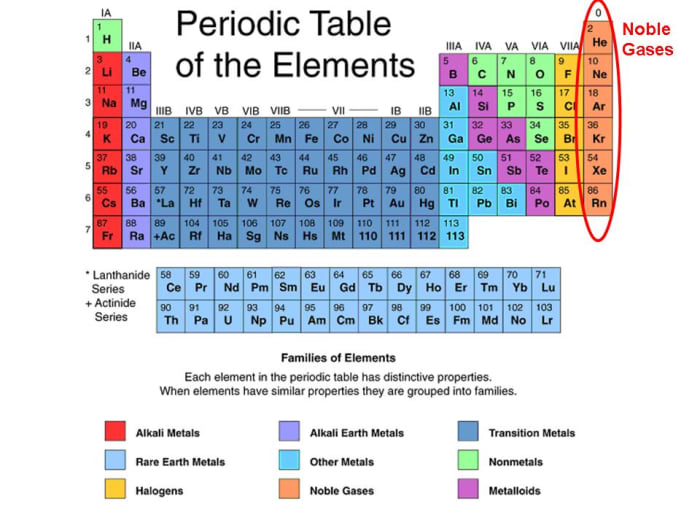
Group 18 The Noble Gases
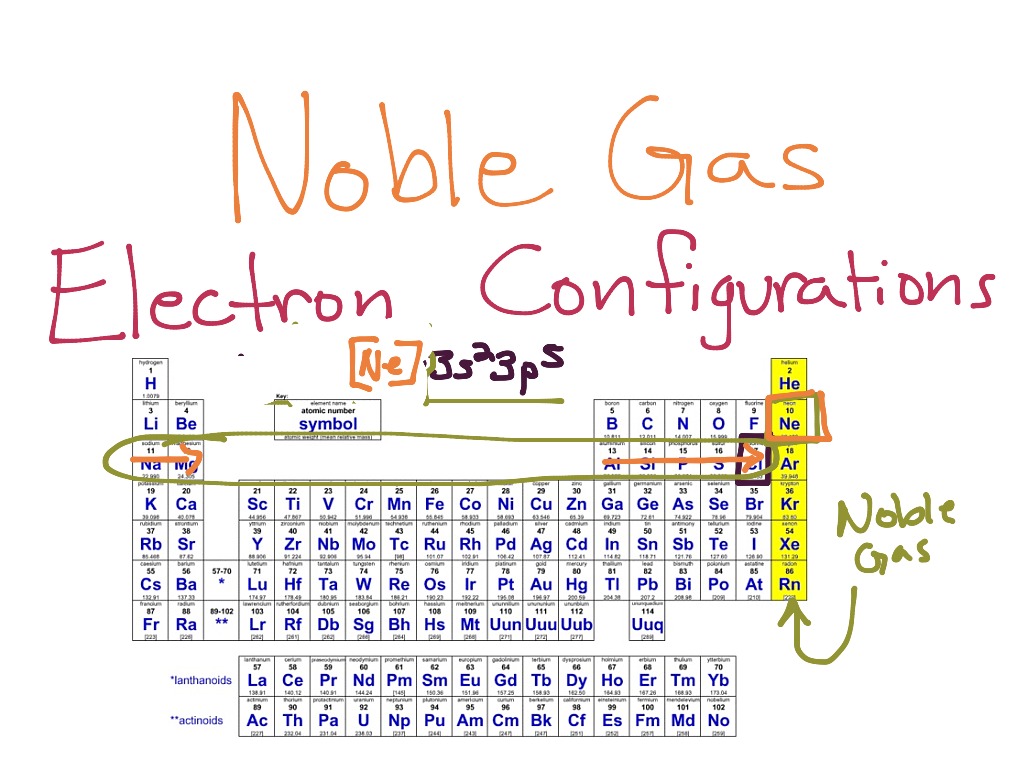
Noble Gas Electron Configurations Electron Configuration, High School
For Helium, That Limit Is Just Two Electrons.
Each F Shell Holds Up To 14 Electrons.
The Aufbau Principle States That Electrons Fill Lower Energy Levels Before Adding To Higher Energy Levels.
Noble Gases, Which Include Helium, Neon, Argon, Krypton, Xenon, And Radon, Have A Full Outer Electron Shell, Making Them Stable And Unreactive.
Related Post: