Valence Electron Pattern For A Carbon Atom
Valence Electron Pattern For A Carbon Atom - As we go down the. Web which diagram shows the valence electron pattern for a carbon atom? Carbon has two valence electrons in the 2s subshell, plus two in the 2p subshell, so its valence electron. Web valence bond theory looks at the interaction between atoms to explain chemical bonds. How many electrons does a carbon. In water, oxygen has a valence of two; These electrons are arranged according to specific rules in different. Web atomic carbon has six electrons: Web valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of. Web because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. Web so whether it's a carbocation or a carbanion, the carbon will begin with four valence electrons. Web the given diagrams of valence electron patterns show that the valence electron pattern that is for a carbon atom is a) a. Two inner shell (core) electrons in the 1 s orbital, and four valence (outer most shell) electrons in the 2. Two inner shell (core) electrons in the 1 s orbital, and four valence (outer most shell) electrons in the 2 s and 2 p orbitals. Web which diagram shows the valence electron pattern for a carbon atom? These electrons are arranged according to specific rules in different. As we go down the. The outer electrons have the highest energy of. The total number of electrons in the last shell after the electron configuration of carbon is called the valence electrons of. Web which diagram shows the valence electron pattern for a carbon atom? These electrons are arranged according to specific rules in different. And in hydrogen chloride, chlorine has a valence of 1. Two inner shell (core) electrons in the. Web atomic carbon has six electrons: The presence of valence electrons can determine the element's chemical prope… The valence configuration of carbon is 2s²2p². Web because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. A) a b) b c) c d) d. Web valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of. With a carbocation the carbon will lose a valence electron and take on a. To find how many valence electrons are in an atom, you can look at the periodic table: In single covalent. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. Web which diagram shows the valence electron pattern for a carbon atom? Diagram with 4 valence electrons (4 dots) why are carbon atoms the building blocks for. Diagram with 4 valence electrons (4 dots) why are carbon atoms the building blocks for life? Web as we go across a period from left to right, we add a proton to the nucleus and an electron to the valence shell with each successive element. Web so whether it's a carbocation or a carbanion, the carbon will begin with four. These electrons are arranged according to specific rules in different. Web the given diagrams of valence electron patterns show that the valence electron pattern that is for a carbon atom is a) a. How many electrons does a carbon. It is one of the two common theories that helps describe the bonding. In a single covalent bond, a shared pair. These electrons are arranged according to specific rules in different. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. Start studying 2.11 review mid. Web valence electrons are the electrons that reside in the outermost energy. Web what is the electron configuration of carbon? The total number of electrons in the last shell after the electron configuration of carbon is called the valence electrons of. Web which diagram shows the valence electron pattern for a carbon atom? In water, oxygen has a valence of two; A) a b) b c) c d) d. In single covalent bonds, typically both atoms in the. Carbon has two valence electrons in the 2s subshell, plus two in the 2p subshell, so its valence electron. Web atomic carbon has six electrons: Web in methane, carbon has a valence of 4; How many electrons does a carbon. Web electronic structures of cations. The valence configuration of carbon is 2s²2p². Diagram with 4 valence electrons (4 dots) why are carbon atoms the building blocks for life? The total number of electrons in the last shell after the electron configuration of carbon is called the valence electrons of. Web what are the valence electrons of carbon? The total number of electrons in carbon is six. Two inner shell (core) electrons in the 1 s orbital, and four valence (outer most shell) electrons in the 2 s and 2 p orbitals. Web because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. With a carbocation the carbon will lose a valence electron and take on a. These electrons are arranged according to specific rules in different. Web which diagram shows the valence electron pattern for a carbon atom?
Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
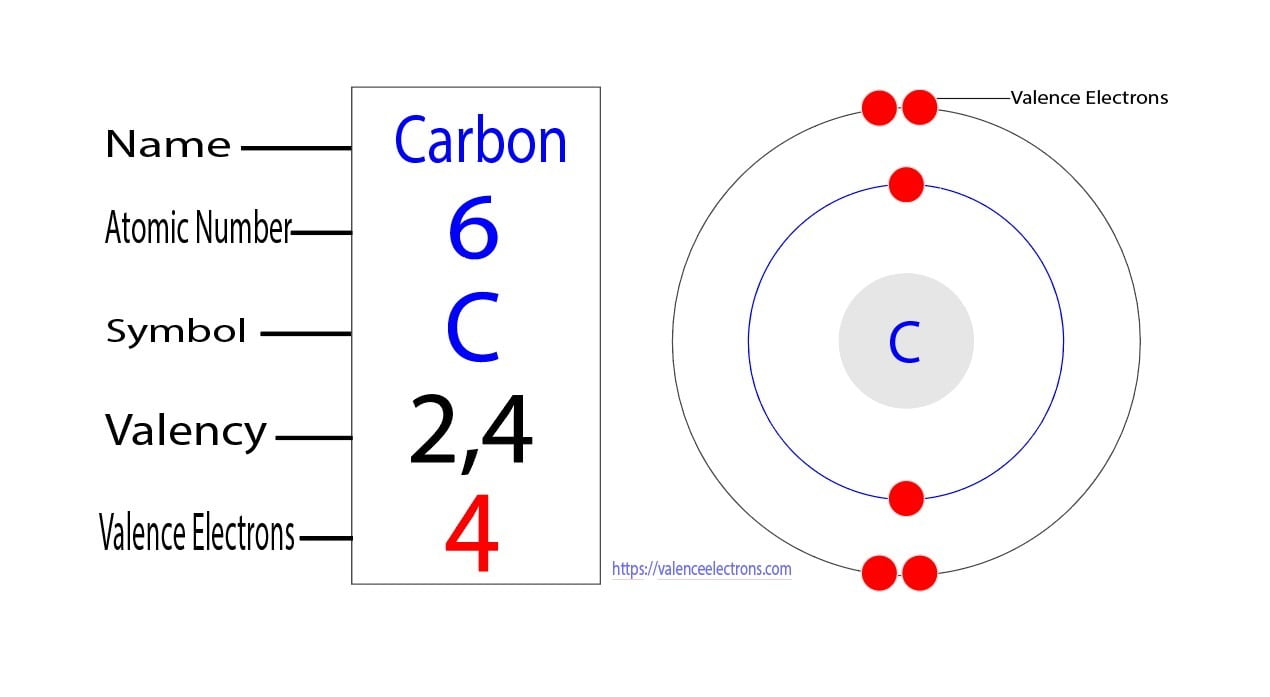
How to Find the Valence Electrons for Carbon(C)?
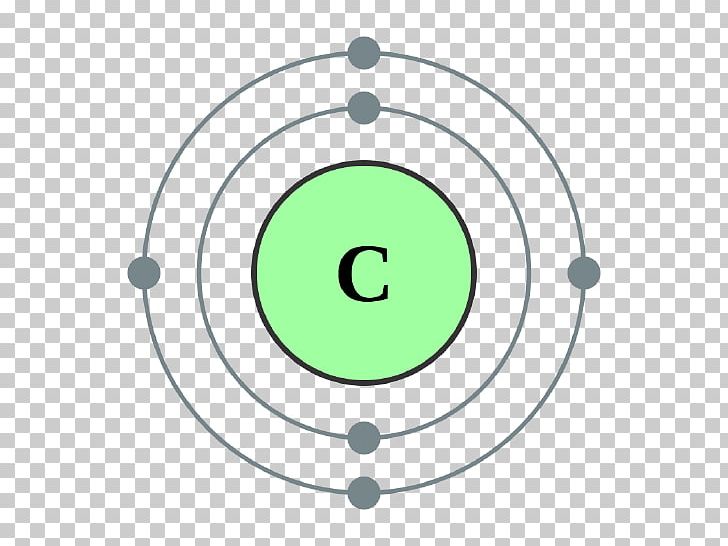
Carbon Electron Shell Diagram
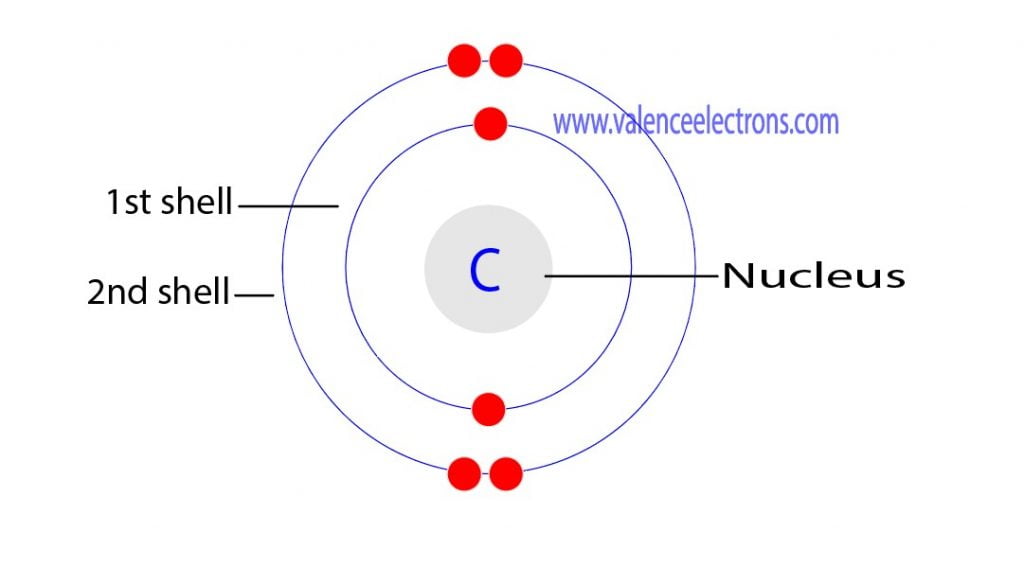
Electron Configuration for Carbon (C, C4−) Full Guide

Valence Electrons Of All Elements Valence Electrons
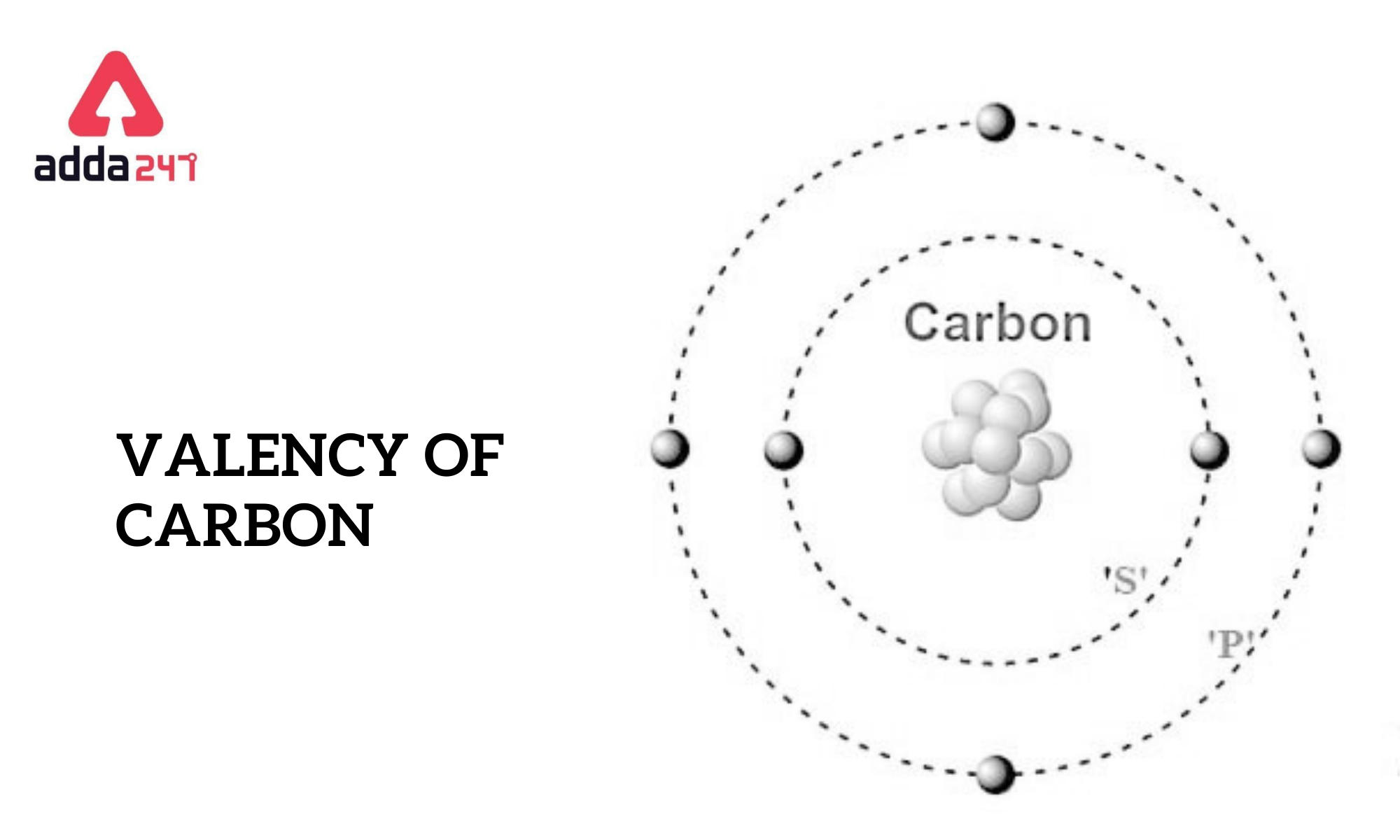
Valency of Carbon Check carbon valency electrons

High diagram shows the valence electron pattern for a carbon atom
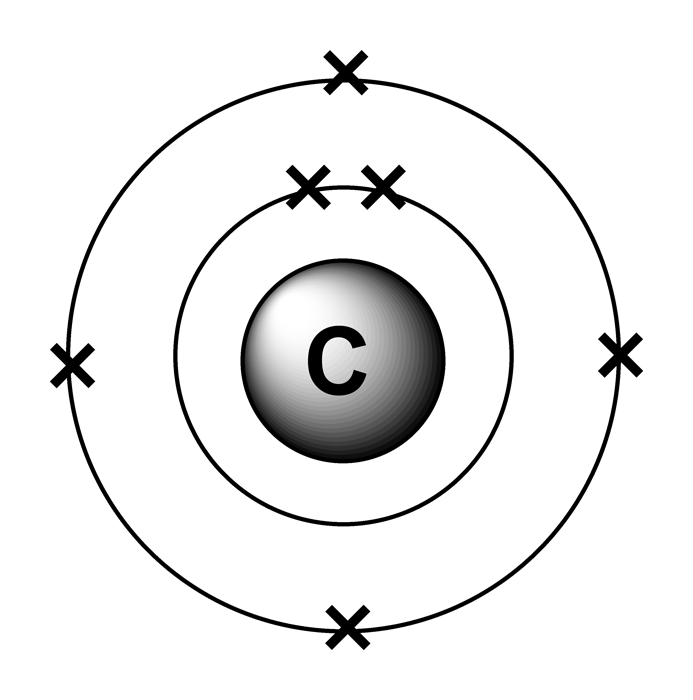
Electron arrangements
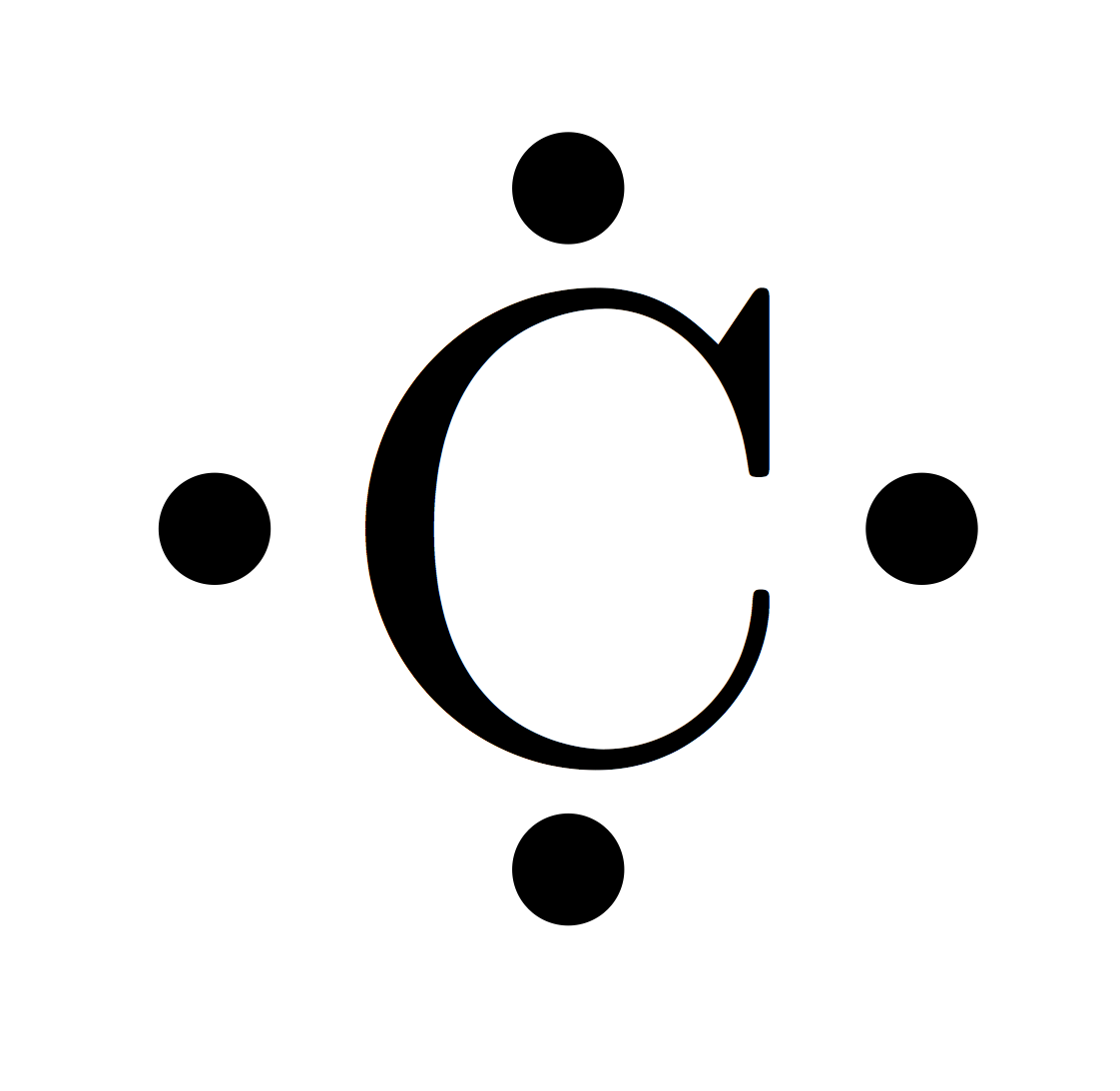
How to Resolve The Valency of Carbon Electronic Configuration

Study these diagrams. The dots represent valence electrons orbiting the
In Water, Oxygen Has A Valence Of Two;
Web Valence Electrons Are The Electrons That Reside In The Outermost Energy Level Of An Atom And Are, Therefore, The Most Accessible For The Formation Of.
Web Valence Bond Theory Looks At The Interaction Between Atoms To Explain Chemical Bonds.
And In Hydrogen Chloride, Chlorine Has A Valence Of 1.
Related Post: