Typical Bonding Pattern For Nitrogen
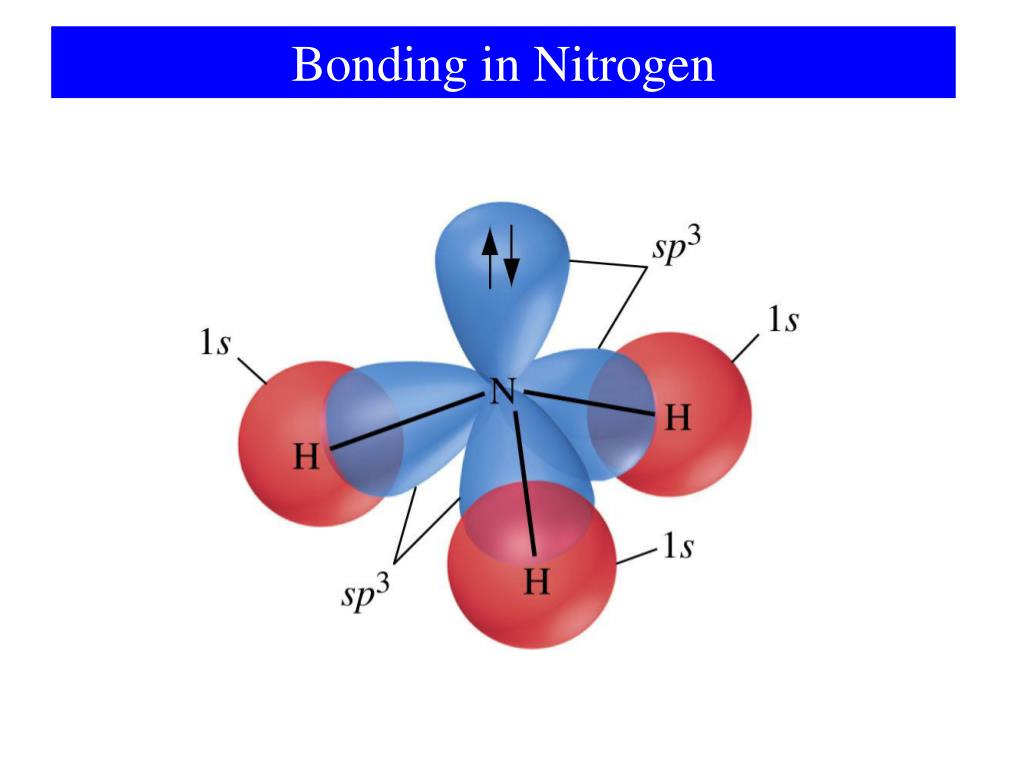
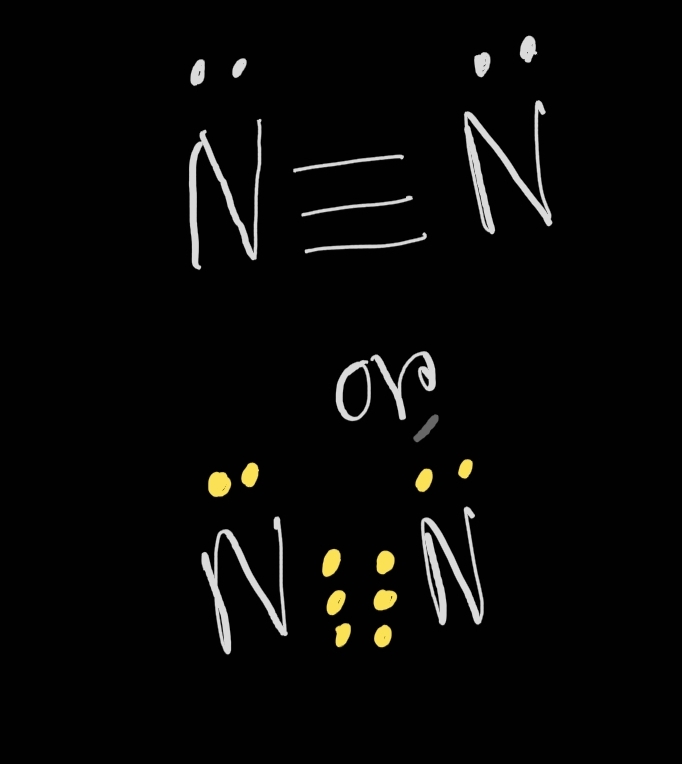
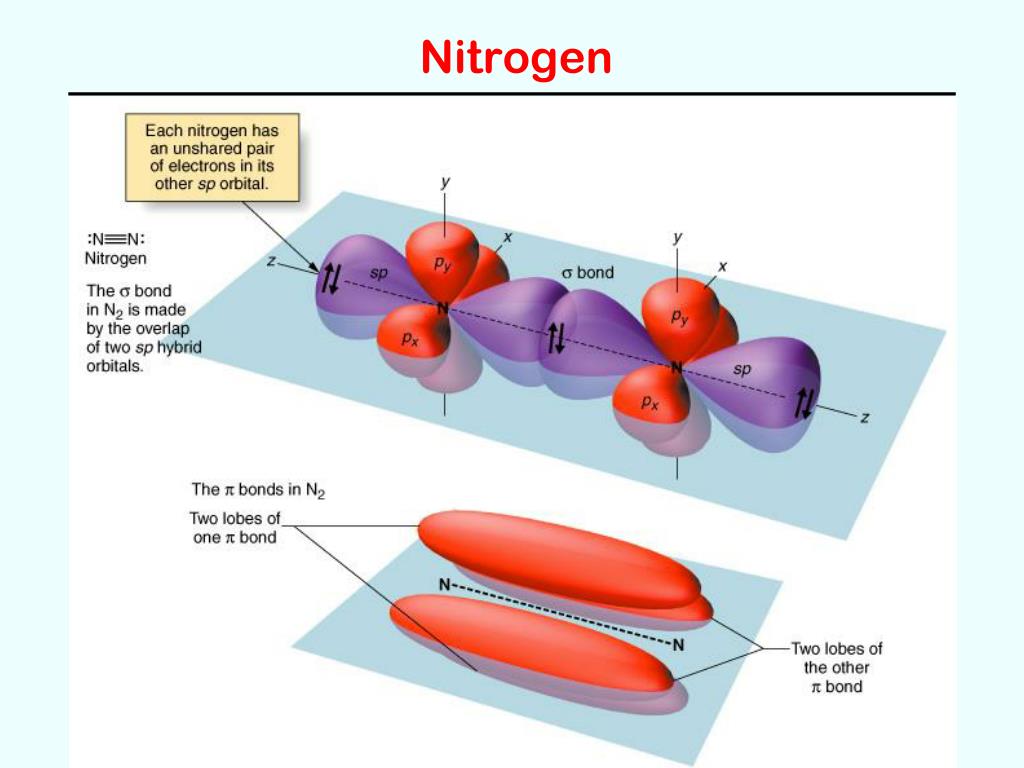
Typical Bonding Pattern For Nitrogen - Common bonding patterns for nitrogen: In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs. Web this video reviews the common bonding patterns of hydrogen, boron, carbon, nitrogen, oxygen, and the halogens, as seen in organic chemistry. It can also form 4 covalent bonds by sharing all its valence electrons, but this would give it a positive charge. The two nitrogen atoms are bonded together by a triple bond, consisting of a σ σ and two π π bonds. 1 bond, 0 lone pairs. So, the typical bonding patterns for a neutral nitrogen atom in a molecule or polyatomic ion are: Which of the options below show typical bonding patterns for a neutral nitrogen atom in a molecule or polyatomic ion? One of the familiar bonding patterns is seen in molecular nitrogen (n₂), where each nitrogen atom has a triple bond with the other, following the octet. Use the periodic table to explain why your answer makes sense. Web this video reviews the common bonding patterns of hydrogen, boron, carbon, nitrogen, oxygen, and the halogens, as seen in organic chemistry. Finally, it can also form two bonds as it carries two unshared electron pairs and a negative charge. Common bonding patterns for nitrogen: In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone. If it has four bonds (and no lone pair), it has a formal charge of +1. One of the familiar bonding patterns is seen in molecular nitrogen (n₂), where each nitrogen atom has a triple bond with the other, following the octet. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. Placing. It can also form 4 covalent bonds by sharing all its valence electrons, but this would give it a positive charge. Nitrogen has two major bonding patterns, both of which fulfill the octet rule: Web a neutral nitrogen atom in a molecule or polyatomic ion typically displays several common bonding patterns due to its five valence electrons as it resides. 0 bonds, 0 lone pairs. Three bonds and one lone pair, or four bonds and a positive formal charge. Phosphorus typically forms five bonds without a formal charge. In ammonia the nitrogen atom is. The valence electrons of nitrogen in its compounds are all sp³ hybridized orbitals. Common bonding patterns for organic chemistry is shared under a not declared license and was authored, remixed, and/or curated by libretexts. If it has four bonds (and no lone pair), it has a formal charge of +1. Are different from the typical patterns. Nitrogen has two major bonding patterns, both of which fulfill the octet rule: Web nitrogen can form. Having four valence electrons, it can form four bonds. Drawing structural formulas is a good starting point for a novice organic chemist, and works when dealing with small, simple structures. This way the nitrogen has a filled outer shell and the hydrogens have two electrons to fill their shells. In ammonia the nitrogen atom is. International journal of quantum chemistry. Which of the options below show typical bonding patterns for a neutral nitrogen atom in a molecule or polyatomic ion? Three bonds and one lone pair, or four bonds and a positive formal charge. At a pressure of 115 gpa, the bond. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. If. International journal of quantum chemistry 1983, 24 (6) ,. Are different from the typical patterns. Drawing structural formulas is a good starting point for a novice organic chemist, and works when dealing with small, simple structures. Ab initio and semiempirical calculations of the static potential for electron scattering off the nitrogen molecule. Use the periodic table to explain why your. This is an ammonia molecule with the formula, nh3. The two nitrogen atoms are bonded together by a triple bond, consisting of a σ σ and two π π bonds. Common bonding patterns for organic chemistry is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Nitrogen is occasionally also seen with two bonds, two. Seven electrons allow for one bond. 0 bonds, 1 lone pair. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. 3.circle the structures below that represent a typical bonding pattern for nitrogen. The journal of chemical physics 1972, 57 (11) ,. Having four valence electrons, it can form four bonds. 1 bond, 0 lone pairs. Web a neutral nitrogen atom in a molecule or polyatomic ion typically displays several common bonding patterns due to its five valence electrons as it resides in group 15 of the periodic table. Web the simplest compound of nitrogen is molecular nitrogen, n2 n 2. Web it can form 3 covalent bonds by sharing its 3 unpaired electrons with other atoms and still have a lone pair of electrons left. It can also form four bonds by bearing a positive charge, in which case it carries no unshared electrons. At a pressure of 115 gpa, the bond. One lone pair four bonds; Drawing structural formulas is a good starting point for a novice organic chemist, and works when dealing with small, simple structures. Three bonds and one lone pair, or four bonds and a positive formal charge. With three valence electrons, it forms three bonds. Use the periodic table to explain why your answer makes sense. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs. If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. International journal of quantum chemistry 1983, 24 (6) ,.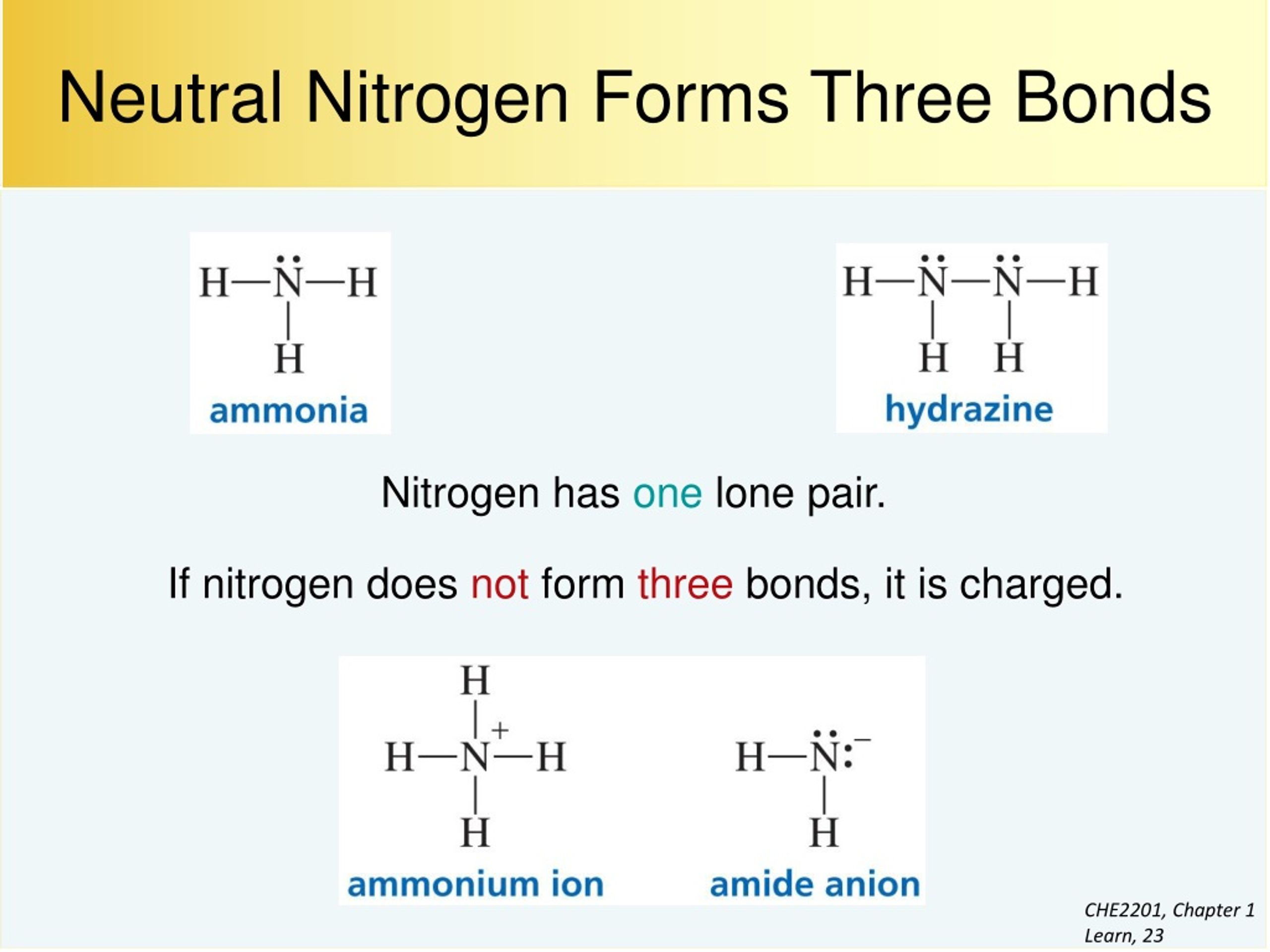
How Many Bonds Does Nitrogen Form Asking List
How Many Bonds Does Nitrogen Form Asking List
N2 Lewis Structure ,Valence Electrons,Formal Charge,Polar or Non polar
PPT Chemical Bonding and Molecular Structure (Ch. 10) PowerPoint

Bonding configurations for nitrogen atoms in NCs. [Reprinted with

SOLVED Which of the options below show typical bonding patterns for a
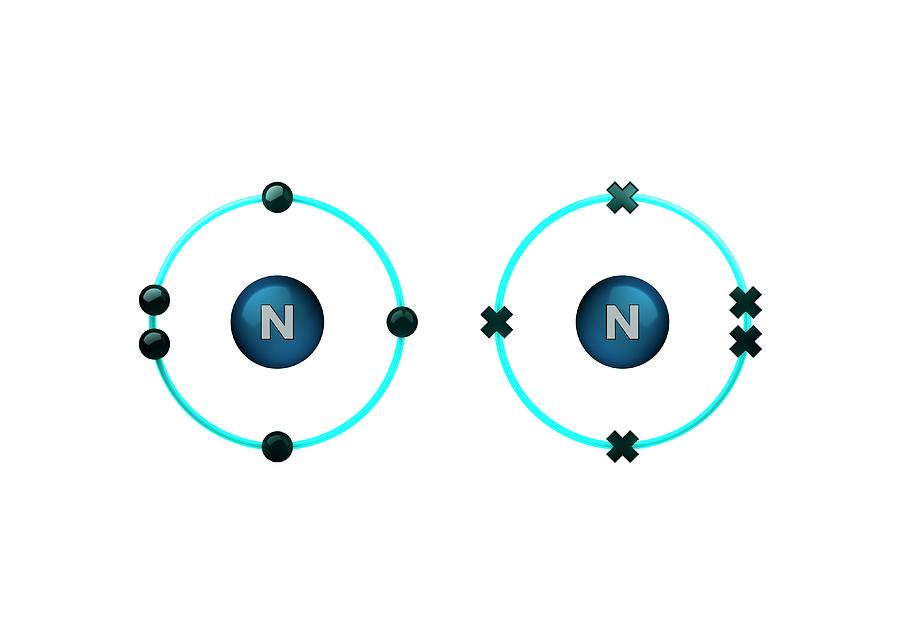
Bond Formation In Nitrogen Molecule Photograph by

Nitrogen Electron Configuration (N) with Orbital Diagram
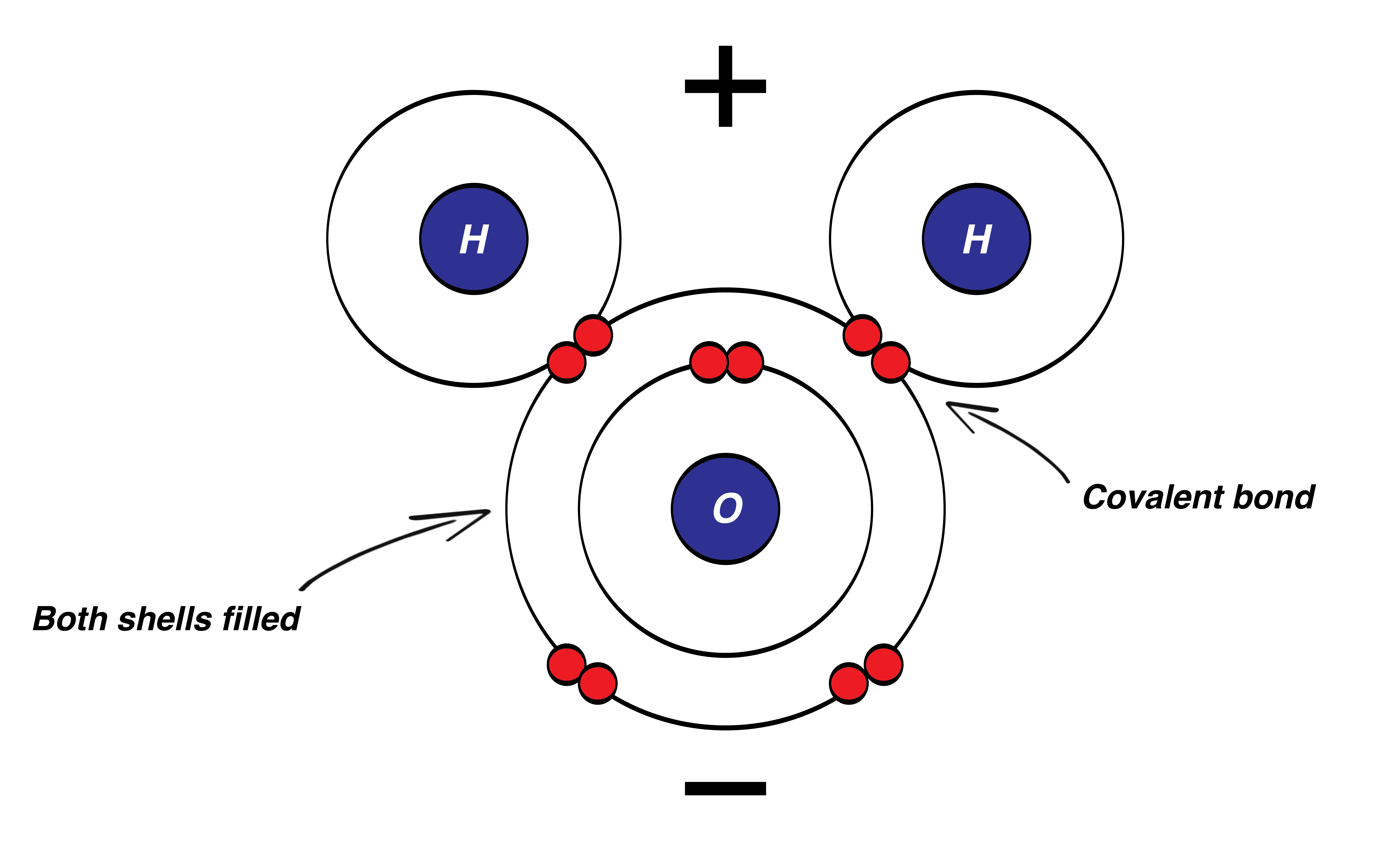
Bohr Diagram For Nitrogen

How to write the Electronic Configuration of Nitrogen Chemical
If It Has Four Bonds (And No Lone Pair), It Has A Formal Charge Of +1.
• Usually P Bonds To Four Oxygen Atoms.
One Of The Familiar Bonding Patterns Is Seen In Molecular Nitrogen (N₂), Where Each Nitrogen Atom Has A Triple Bond With The Other, Following The Octet.
3.Circle The Structures Below That Represent A Typical Bonding Pattern For Nitrogen.
Related Post: