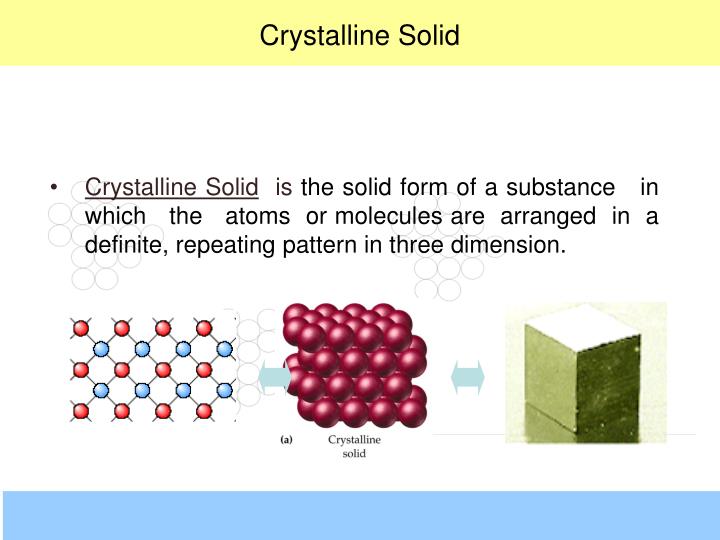
Solids That Have A Repeating Pattern Of Atoms Are Called
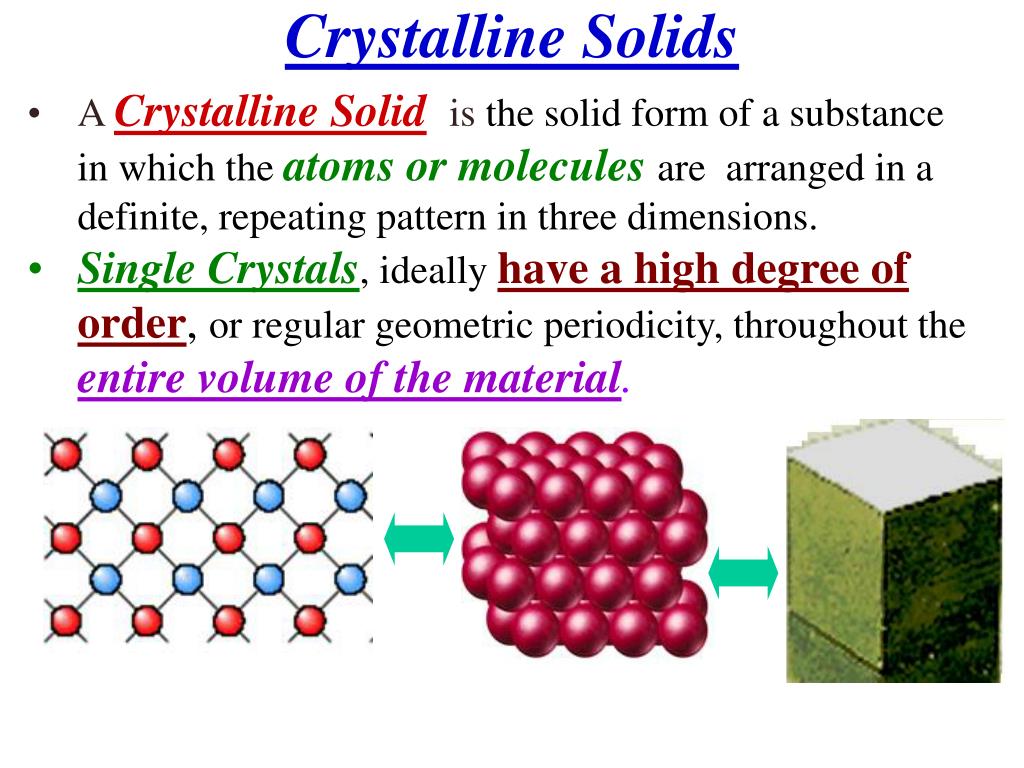
Solids That Have A Repeating Pattern Of Atoms Are Called - The unit cell with all sides the. Web crystalline solids, or crystals, are regarded as true solids. minerals are crystalline solids. Web in a crystalline solid, the atoms, ions, or molecules are arranged in a definite repeating pattern, but occasional defects may occur in the pattern. Web crystalline solids have their molecules or atoms arranged in a repeating pattern, called a lattice structure. The smallest repeating unit of that lattice structure is. There is only one type of amorphous solid. Spaces between the regular particle positions in any array of atoms or ions. Web in crystalline solids, the atoms are arranged in a specific pattern. Web when most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or molecules are arranged in a definite. The unit cell may have more atoms than the empirical formula of a substance because the geometric shape may require several atoms. Web crystalline solids, or crystals, are regarded as true solids. minerals are crystalline solids. The unit cell may have more atoms than the empirical formula of a substance because the geometric shape may require several atoms. Web crystalline solids differ from amorphous solids in that crystalline solids have _____. Several types of defects are. The characteristic angles do not depend. Web a pure metal is a crystalline solid with metal atoms packed closely together in a repeating pattern. Solid composed of positive and negative ions held together by strong electrostatic attractions. Web crystalline solids, or crystals, are regarded as true solids. minerals are crystalline solids. Some of the properties of metals in general, such as their malleability and. The characteristic. Spaces between the regular particle positions in any array of atoms or. Web when most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or molecules are arranged in a definite. The smallest repeating part of this pattern is called the unit cell. The characteristic angles do not depend on the size of. Several types of defects are. Web crystalline solids differ from amorphous solids in that crystalline solids have _____. The smallest repeating unit of that lattice structure is. Web learn the definition of solids that have a repeating pattern of atoms, also known as crystals, and other terms related to minerals, rocks, and rock cycle. The characteristic angles do not depend. Solid in which the particles are arranged in a definite repeating pattern. Web crystalline solids have their molecules or atoms arranged in a repeating pattern, called a lattice structure. Common table salt is one example of this kind of solid. Web when most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or. Web a pure metal is a crystalline solid with metal atoms packed closely together in a repeating pattern. Solid in which the particles are arranged in a definite repeating pattern. The smallest repeating part of this pattern is called the unit cell. Some of the properties of metals in general, such as their malleability and. The unit cell with all. Solid composed of positive and negative ions held together by strong electrostatic attractions. Some of the properties of metals in general, such as their malleability and. Common table salt is one example of this kind of solid. Web when most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or molecules are arranged. The smallest repeating unit of that lattice structure is. Web crystalline solids differ from amorphous solids in that crystalline solids have _____. Web learn the definition of solids that have a repeating pattern of atoms, also known as crystals, and other terms related to minerals, rocks, and rock cycle. A crystal of nacl (see figure 10.13 “properties of solids”) is. The characteristic angles do not depend on the size of the. A crystal of nacl (see figure 10.13 “properties of solids”) is one example: Common table salt is one example of this kind of solid. Web in a crystalline solid, the atoms, ions, or molecules are arranged in a definite repeating pattern, but occasional defects may occur in the pattern.. The smallest repeating unit of that lattice structure is. Some of the properties of metals in general, such as their malleability and. The characteristic angles do not depend on the size of the. Solid in which the particles are arranged in a definite repeating pattern. Web when most liquids are cooled, they eventually freeze and form crystalline solids, solids in. A crystal of nacl (see figure 10.13 “properties of solids”) is one example: Web crystalline solids differ from amorphous solids in that crystalline solids have _____. The unit cell with all sides the. Web a pure metal is a crystalline solid with metal atoms packed closely together in a repeating pattern. Spaces between the regular particle positions in any array of atoms or. Spaces between the regular particle positions in any array of atoms or ions. Common table salt is one example of this kind of solid. Web crystalline solids have their molecules or atoms arranged in a repeating pattern, called a lattice structure. Web crystalline solids, or crystals, are regarded as true solids. minerals are crystalline solids. The smallest repeating unit of that lattice structure is. Some of the properties of metals in general, such as their malleability and. Solid in which the particles are arranged in a definite repeating pattern. Several types of defects are. The characteristic angles do not depend on the size of the. There is only one type of amorphous solid. Solid in which the particles are arranged in a definite repeating pattern.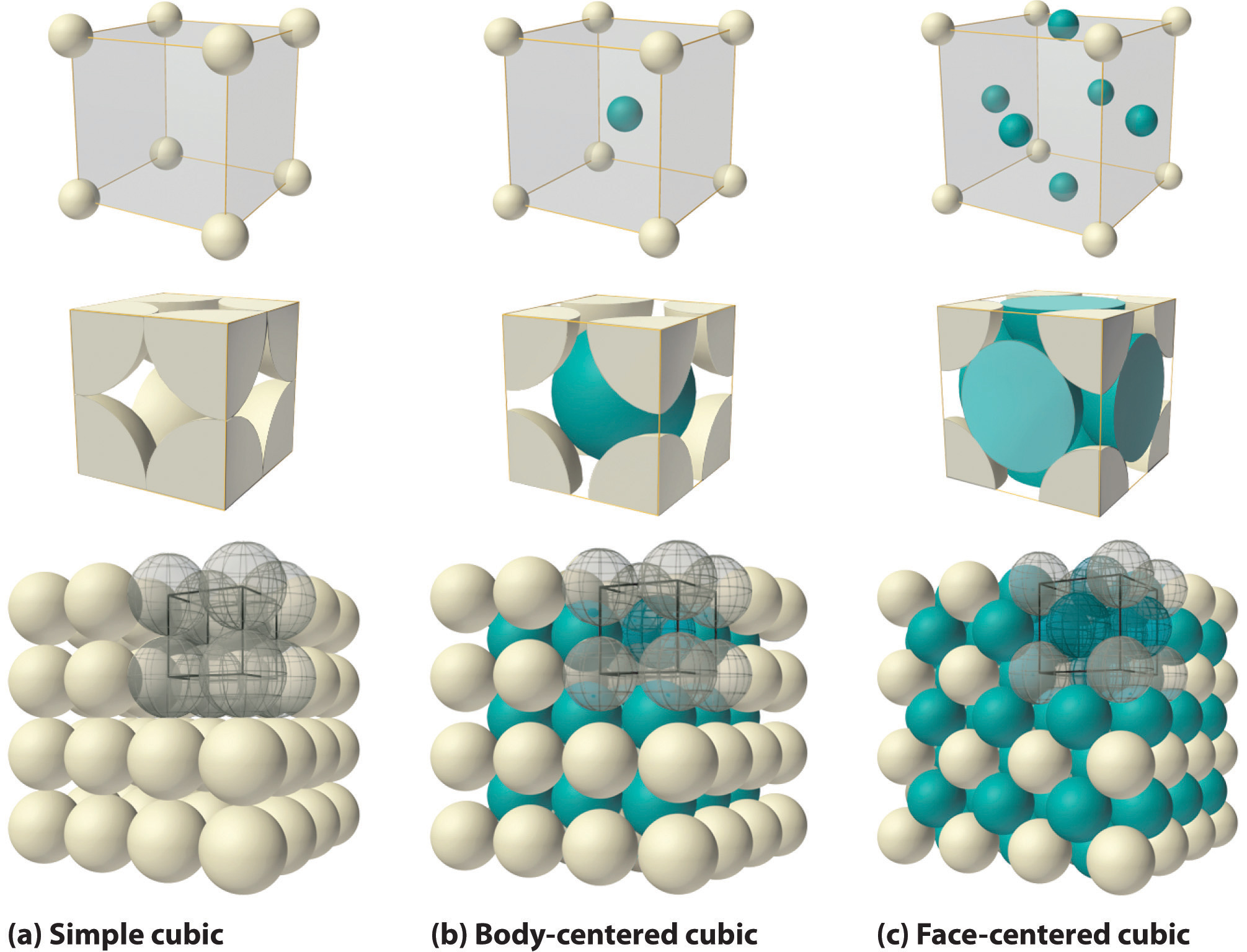
12.2 The Arrangement of Atoms in Crystalline Solids Chemistry LibreTexts

11.7 Structure of Solids Chemistry LibreTexts
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free
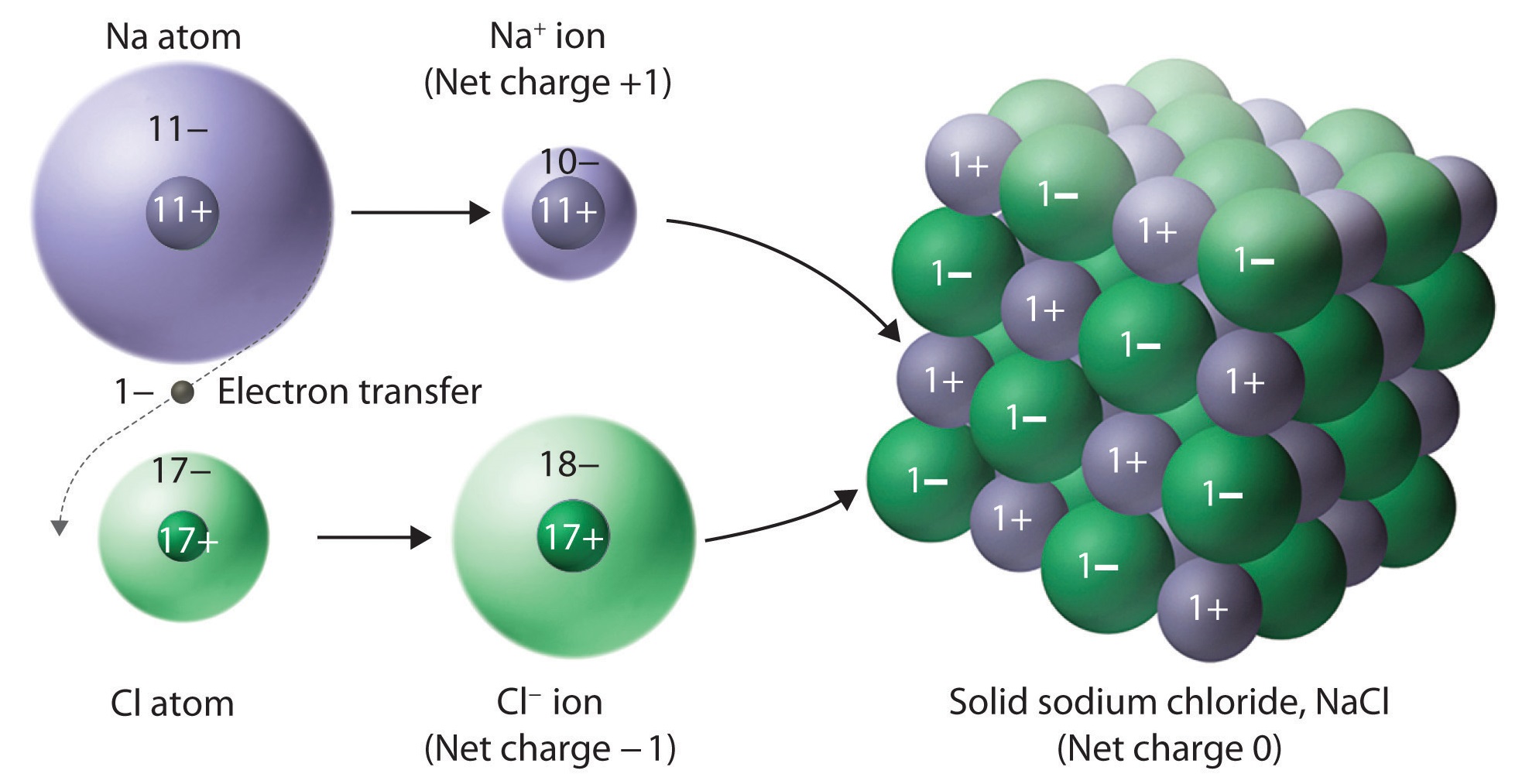
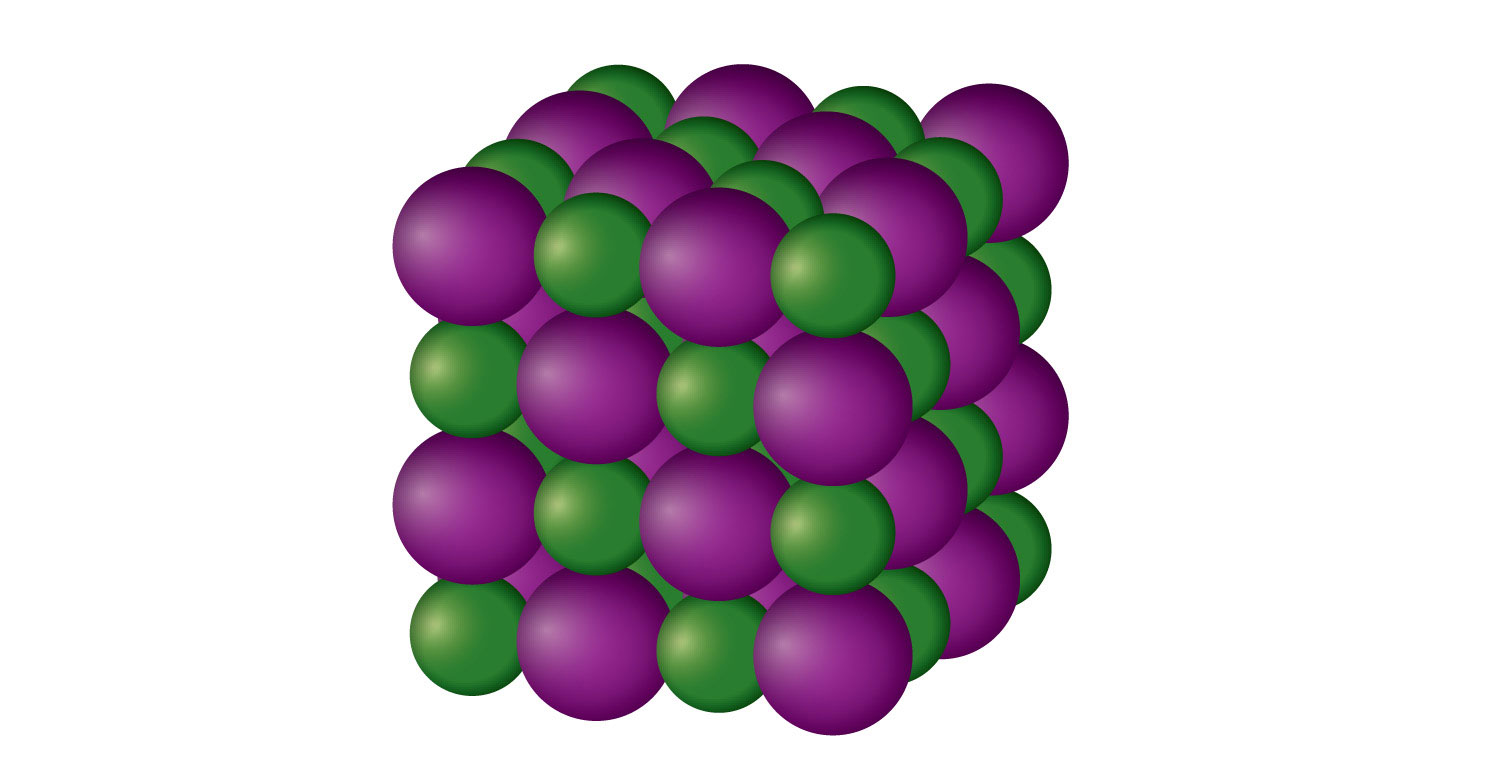
What structural units make up ionic solids? Socratic
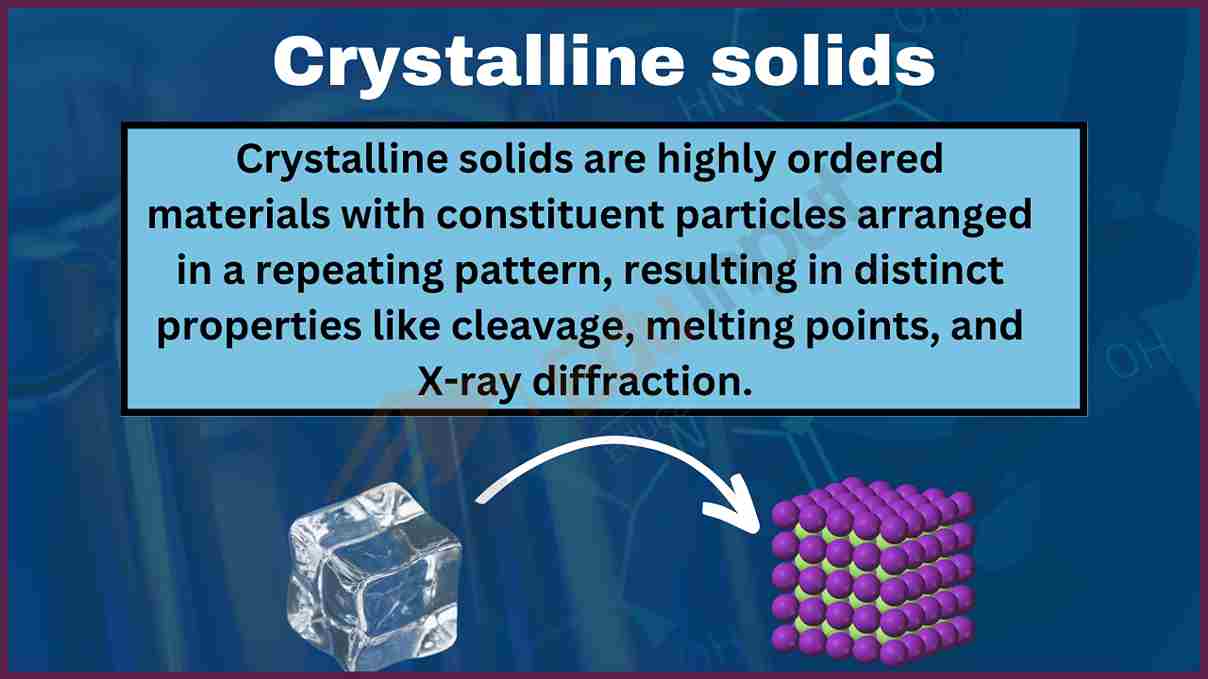
Crystalline solids Properties, types, examples use

Amorphous Solid Easy Science Easy science, Ap chemistry, Flashcards
PPT CHAPTER 1 Atoms and bonding PowerPoint Presentation ID868644
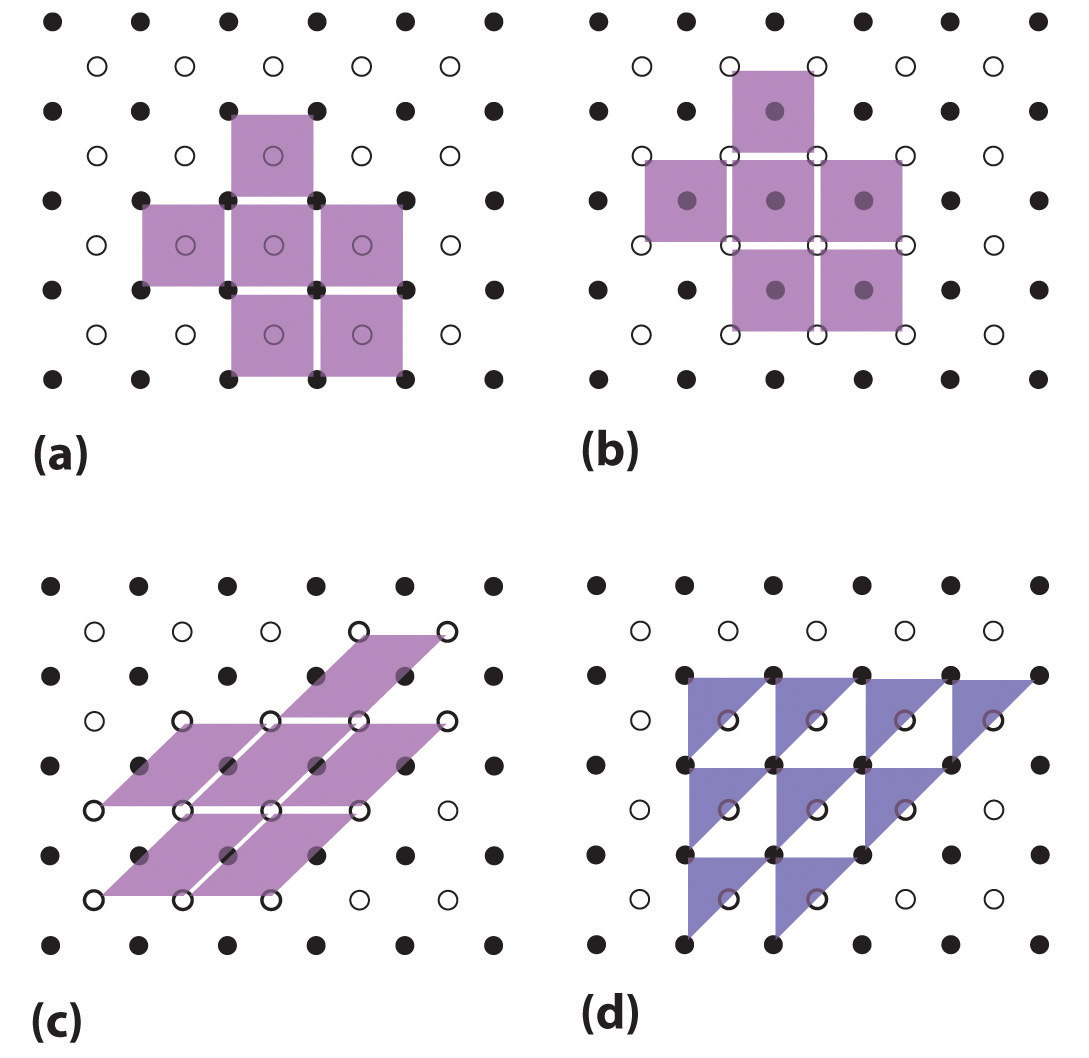
11.7 Structure of Solids Chemistry LibreTexts

11. What is the solid form of a mineral produced by a repeating pattern
Solids
Web When Most Liquids Are Cooled, They Eventually Freeze And Form Crystalline Solids, Solids In Which The Atoms, Ions, Or Molecules Are Arranged In A Definite.
Web In A Crystalline Solid, The Atoms, Ions, Or Molecules Are Arranged In A Definite Repeating Pattern, But Occasional Defects May Occur In The Pattern.
The Unit Cell May Have More Atoms Than The Empirical Formula Of A Substance Because The Geometric Shape May Require Several Atoms.
Web In Crystalline Solids, The Atoms Are Arranged In A Specific Pattern.
Related Post: