Resonance Structure Patterns
Resonance Structure Patterns - Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: 2) do not break single bonds. Structures with all atoms having complete octet are most important. I'm gonna highlight it in magenta, that lone. Web all resonance structures must follow the rules of writing lewis structures. Web don’t exceed an octet for 2nd row elements. Carbon can have less than an octet in a resonance structure, but n,o, halogens cannot have less than an octet. Web 3 rules of resonance structures. Web examples showing how different types of bond configurations can be represented using resonance structures. Web learn how to come up with resonance structures by learning key arrow patterns showing you which electrons can move, and where to move them. Major and minor resonance structures. Carbon can have less than an octet in a resonance structure, but n,o, halogens cannot have less than an octet. The skeleton of the structure can not. 2) do not break single bonds. Web 3 rules of resonance structures. Structures with all atoms having complete octet are most important. The hybridization of the structure must stay the same. Web all resonance structures must follow the rules of writing lewis structures. Web 3 rules of resonance structures. 2) do not break single bonds. It explains how to identify the major resonance contr. Web resonance structures are various forms of the same molecule where the electrons have transferred from one region to another. Major and minor resonance structures. Carbon can have less than an octet in a resonance structure, but n,o, halogens cannot have less than an octet. I'm gonna highlight it in magenta,. Web 3 rules of resonance structures. The skeleton of the structure can not. Resonance is a mental exercise and method. Web all resonance structures must follow the rules of writing lewis structures. I'm gonna highlight it in magenta, that lone. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Carbon can have less than an octet in a resonance structure, but n,o, halogens. Negative charge is most stable on most electronegative element. It explains how to identify the major resonance contr. Created by jay.watch the next lesson:. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web key arrow patterns in resonance structures. Created by jay.watch the next lesson:. Web 3 rules of resonance structures. Negative charge is most stable on most electronegative element. 2) do not break single bonds. It explains how to identify the major resonance contr. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. Carbon can have less than an octet in a resonance structure, but n,o, halogens cannot have less than an octet. The skeleton of the structure can. Web resonance structures are various forms of the same molecule where the electrons have transferred from one region to another. I'm gonna highlight it in magenta, that lone. Created by jay.watch the next lesson:. Web draw the resonance structures of molecules or ions that exhibit delocalization. Resonance is a mental exercise and method. 2) do not break single bonds. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Web resonance structures are various forms of the same molecule where the electrons have transferred from one region to another. Let's look at a few of the patterns for drawing resonance structures, and the first pattern we're gonna look at,. It explains how to identify the major resonance contr. Web key arrow patterns in resonance structures. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Web learn how to come up with resonance structures by learning key arrow patterns showing you which electrons can move, and where to move them. Structures with all atoms having complete octet are most important. Created by jay.watch the next lesson:. The skeleton of the structure can not. And so, here's a lone pair of electrons; Determine the relative stability of resonance structures using a set of rules. I'm gonna highlight it in magenta, that lone. Why are resonance structures important?. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis. The hybridization of the structure must stay the same. Web examples showing how different types of bond configurations can be represented using resonance structures. Let's look at a few of the patterns for drawing resonance structures, and the first pattern we're gonna look at, is a lone pair of electrons next to a pi bond.
How to Draw Resonance Structures Organic Chemistry
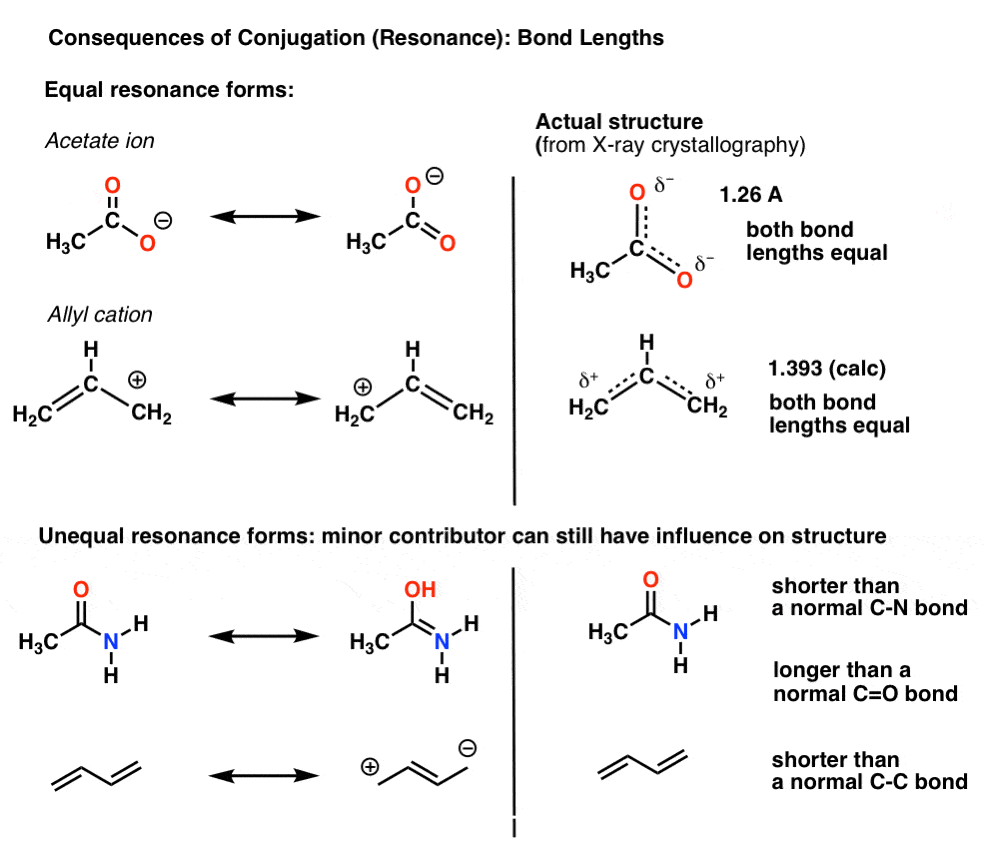
Conjugation And Resonance In Organic Chemistry
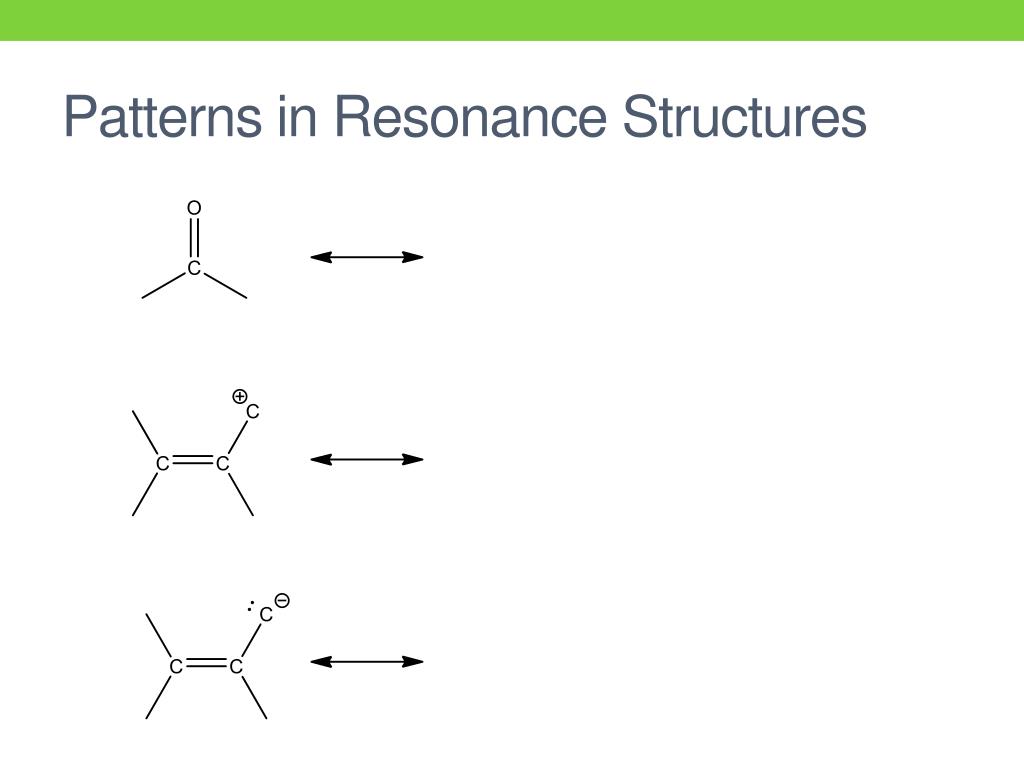
PPT Structure PowerPoint Presentation, free download ID2110530
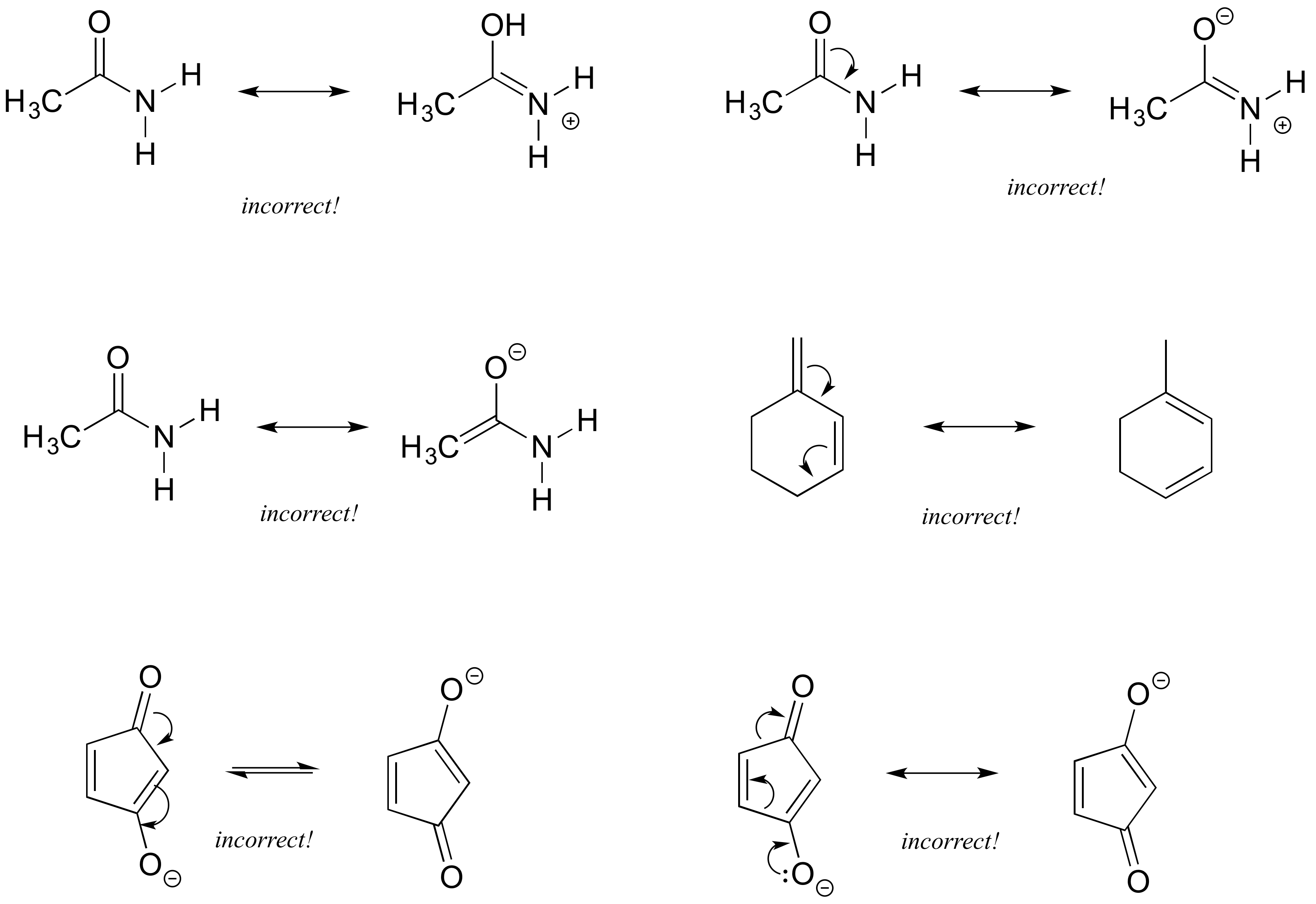
draw the resonance structure of a peptide bond cheaperboschcrevicetool
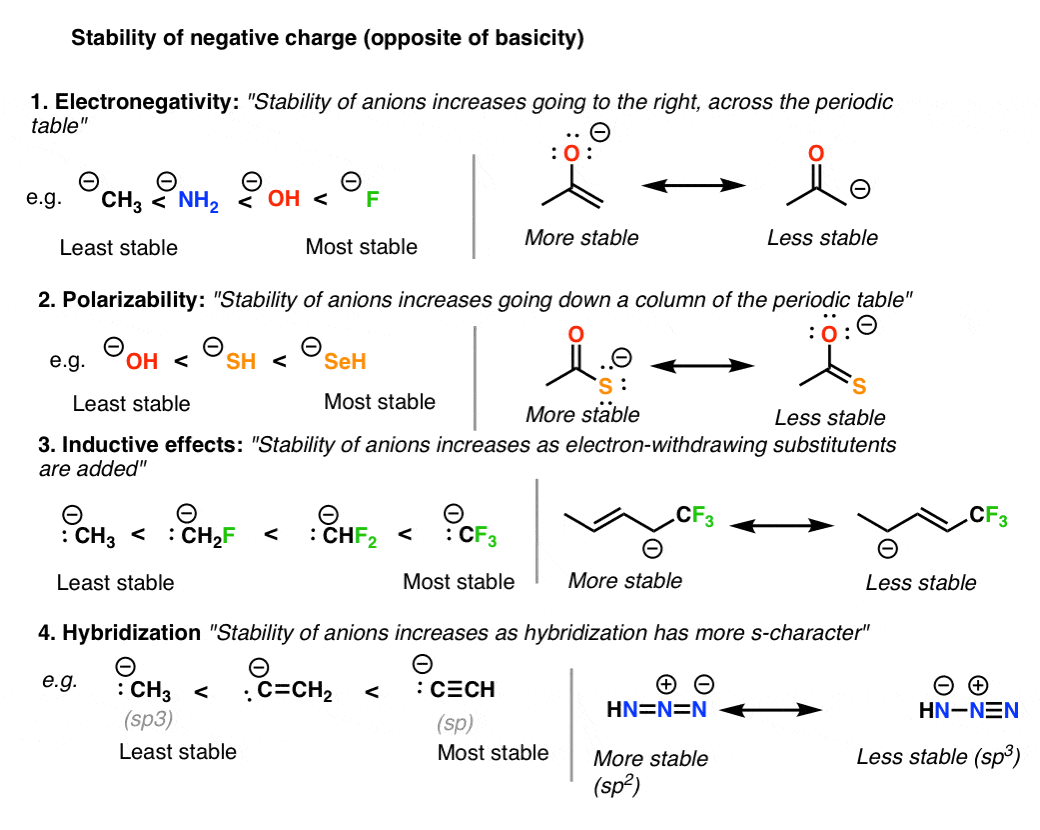
Resonance Structures 4 Rules On How To Evaluate Them, With Practice

6.2. Resonance Organic Chemistry 1 An open textbook

Resonance structure patterns Resonance and acidbase chemistry

Resonance Structures YouTube

resonance structure patterns YouTube

Resonance Structures, Basic Introduction How To Draw The Resonance
Web Draw The Resonance Structures Of Molecules Or Ions That Exhibit Delocalization.
2) Do Not Break Single Bonds.
Web Don’t Exceed An Octet For 2Nd Row Elements.
Web Resonance Structures Are Various Forms Of The Same Molecule Where The Electrons Have Transferred From One Region To Another.
Related Post: