Research Protocol Template
Research Protocol Template - Web research study protocol template. Learn what a protocol is, how it is used in scientific research and review, and how to use the. Web a research protocol is a plan for how a study is run, including the aims, objectives, methods, ethical considerations and data analysis. There is no single protocol template. Web the first drafting of the protocol for a new research project should start from a solid idea with one or more of these goals: The natural history/observational protocol template, the repository protocol template, and the secondary research protocol template. Web it too offers much help and guidance for writing your protocol and conducting your review. Web there are three templates to be used for observational research: Trials is experimenting with a new way of structuring study protocols for randomised trials. Web a protocol is a comprehensive guide to protocol development for unc investigators. They follow the format of typical nih and industry multicenter protocols. Web what are the elements/sections of a research protocol? According to good clinical practice guidelines laid by who, a research protocol should include the. Web a study protocol is an important document that specifies the research plan for a clinical study. Trials is experimenting with a new way of. The simple innovation is to include all. Learn what a protocol is, how it is used in scientific research and review, and how to use the. Web the irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. Web qualitative protocol guidance and template we would find your feedback useful to. Web structured study protocol template | trials. It should be written in detail and researchers should aim to publish. Web a study protocol is an important document that specifies the research plan for a clinical study. Learn what a protocol is, how it is used in scientific research and review, and how to use the. The simple innovation is to. Web the first drafting of the protocol for a new research project should start from a solid idea with one or more of these goals: Learn what a protocol is, how it is used in scientific research and review, and how to use the. Web nih applicants can use a template with instructional and sample text to help write clinical. This template provides guidance on. Web the first drafting of the protocol for a new research project should start from a solid idea with one or more of these goals: Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were developed to assist investigators in preparation of. According to good. Web structured study protocol template | trials. Web the research protocol should provide the information needed for reviewers to determine that the regulatory and human research protection program (hrpp) policy. Web the irb office has developed protocol templates for use by the northwestern university research community to describe research/human research activities. This protocol template is a tool to facilitate the. The natural history/observational protocol template, the repository protocol template, and the. This template provides guidance on. A research protocol is a detailed description of a research project, including its aims, methods, design, safety, and dissemination. Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were developed to assist investigators in. Web what are the elements/sections of a research protocol? Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were developed to assist investigators in preparation of. Learn what a protocol is, how it is used in scientific research and review, and how to use the. Practice this means satisfying itself. Web a protocol is a comprehensive guide to protocol development for unc investigators. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for phase 2 or 3 clinical trials that require investigational new. Web there are three templates to be used for observational research: Sections of interest may include ychapter ii: Web. Web qualitative protocol guidance and template we would find your feedback useful to help us refine this document. Web research study protocol template. According to good clinical practice guidelines laid by who, a research protocol should include the. It should be written in detail and researchers should aim to publish. Web the following resources offer templates for authors to develop. Web there are three templates to be used for observational research: There is no single protocol template. The simple innovation is to include all. Web there are three templates to be used for observational research: This protocol template is a tool to facilitate the development of a research study protocol specifically. Planning a cochrane review z, ychapter. According to good clinical practice guidelines laid by who, a research protocol should include the. Web research study protocol template. Sections of interest may include ychapter ii: Web the irb provides several protocol templates on this page. Trials is experimenting with a new way of structuring study protocols for randomised trials. Web the first drafting of the protocol for a new research project should start from a solid idea with one or more of these goals: Web data and safety monitoring plan (dsmp) template and guidelines (ms word, 37k) and dsmp checklist (ms word, 43k) were developed to assist investigators in preparation of. Practice this means satisfying itself the research protocol,. Web at worse actual patient harm. Web the following resources offer templates for authors to develop a systematic review protocol.
Sample Research Protocol TemplateNEJM Resident 360 Doc Template pdfFiller
Research Protocol Template PDF Risk Survey Methodology

21 Elements of a Research Protocol with Example (WHO Guidelines)

Research protocol template
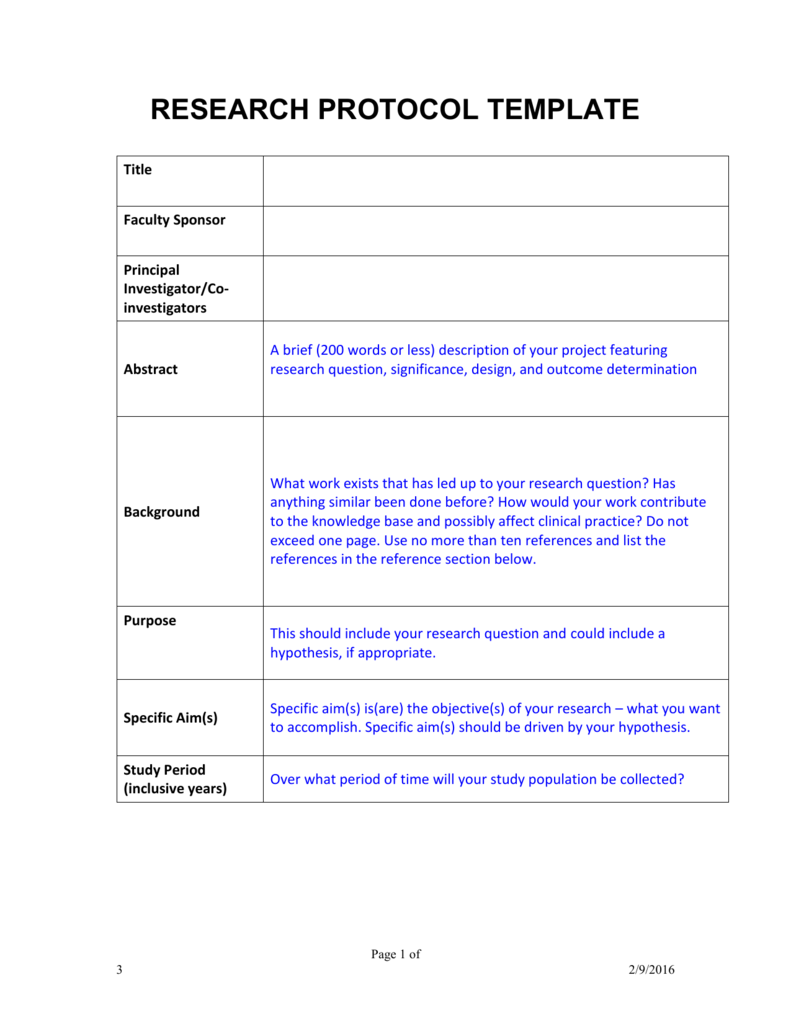
research protocol template

Template Device protocol

Minimal Risk Protocol Template INSTRUCTIONS

Clinical Study Protocol (CSP) Template Clinical Study Templates

Standard Protocol Template NIHR Clinical Research Network

Steps in writing a research protocol for thesis Cosmoderma
Learn What A Protocol Is, How It Is Used In Scientific Research And Review, And How To Use The.
Web A Study Protocol Is An Important Document That Specifies The Research Plan For A Clinical Study.
Web Qualitative Protocol Guidance And Template We Would Find Your Feedback Useful To Help Us Refine This Document.
Web A Protocol Is A Full Description Of A Research Study That Guides The Members Of The Research Team And Monitors The Study's Progress And Outcomes.
Related Post:
