Psur Template
Psur Template - The psur is not mandatory for all the. A periodic safety update report (psur) is a systematic review of the global safety data of an approved medicine that becomes available to you during a. Web what is the psur? Web periodic safety update report [iso 13485 templates] iso 13485 / mdr document template: Within one psur reporting period several updates can be. One or two years is reached. How to submit your periodic safety update report report (psur) or periodic benefit risk. Web guidance on periodic safety update report (psur) according to regulation (eu) 2017/745 (mdr). Web a free resource to help you document all necessary components that must be included in your periodic safety update report (psur) in accordance with the requirements of eu. Web psur file template. The main template provides detailed guidance on how to complete each section of the psur. Web periodic safety update report [iso 13485 templates] iso 13485 / mdr document template: Web periodic safety update reports (psurs) for medicinal products. The psur is not mandatory for all the. Web guidance on periodic safety update report (psur) according to regulation (eu) 2017/745 (mdr). One or two years is reached. Cover page of periodic safety update report (psur) (rev 1). Periodic safety update report contains all. The psur is not mandatory for all the. Web psur file template. How to submit your periodic safety update report report (psur) or periodic benefit risk. Web a psur is generally not intended for the notification of urgent new safety or efficacy information, which may have an important public health impact. Web this page includes information on periodic safety update reports (psurs), psur submission requirements, psur single assessment procedures (psusas) and the.. Web psur file template. Web guidance on periodic safety update report (psur) according to regulation (eu) 2017/745 (mdr). Periodic adverse drug experience report (pader), u.s. Periodic safety update report contains all. How to submit your periodic safety update report report (psur) or periodic benefit risk. Web the psur reporting period closes when the timeline stated in article 86 and 81 i.e. Web psur file template. The psur is not mandatory for all the. Web what is the psur? Periodic safety update report contains all. Web periodic safety update reports (psurs) for medicinal products. Web periodic safety update report [iso 13485 templates] iso 13485 / mdr document template: Web a psur is generally not intended for the notification of urgent new safety or efficacy information, which may have an important public health impact. Web the psur reporting period closes when the timeline stated in article. Web this page includes information on periodic safety update reports (psurs), psur submission requirements, psur single assessment procedures (psusas) and the. The main template provides detailed guidance on how to complete each section of the psur. One or two years is reached. Web psur file template. Cover page of periodic safety update report (psur) (rev 1). Web a free resource to help you document all necessary components that must be included in your periodic safety update report (psur) in accordance with the requirements of eu. The psur is not mandatory for all the. Cover page of periodic safety update report (psur) (rev 1). Web psur file template. Web periodic safety update reports (psurs) for medicinal products. Web guidance on periodic safety update report (psur) according to regulation (eu) 2017/745 (mdr). Web periodic safety update reports (psurs) for medicinal products. The main template provides detailed guidance on how to complete each section of the psur. Periodic adverse drug experience report (pader), u.s. Web template for cover page for psur submission. Web the psur reporting period closes when the timeline stated in article 86 and 81 i.e. Web what is the psur? One or two years is reached. Web guidance on periodic safety update report (psur) according to regulation (eu) 2017/745 (mdr). The psur is not mandatory for all the. Web periodic safety update report [iso 13485 templates] iso 13485 / mdr document template: Periodic adverse drug experience report (pader), u.s. Web psur file template. The main template provides detailed guidance on how to complete each section of the psur. How to submit your periodic safety update report report (psur) or periodic benefit risk. Web periodic safety update reports (psurs) for medicinal products. Web the psur reporting period closes when the timeline stated in article 86 and 81 i.e. Web a free resource to help you document all necessary components that must be included in your periodic safety update report (psur) in accordance with the requirements of eu. Web guidance on periodic safety update report (psur) according to regulation (eu) 2017/745 (mdr). The psur is not mandatory for all the. Web template for cover page for psur submission. One or two years is reached. Web what is the psur? A periodic safety update report (psur) is a systematic review of the global safety data of an approved medicine that becomes available to you during a. Within one psur reporting period several updates can be.
Periodic Safety Update Report (PSUR) Procedure
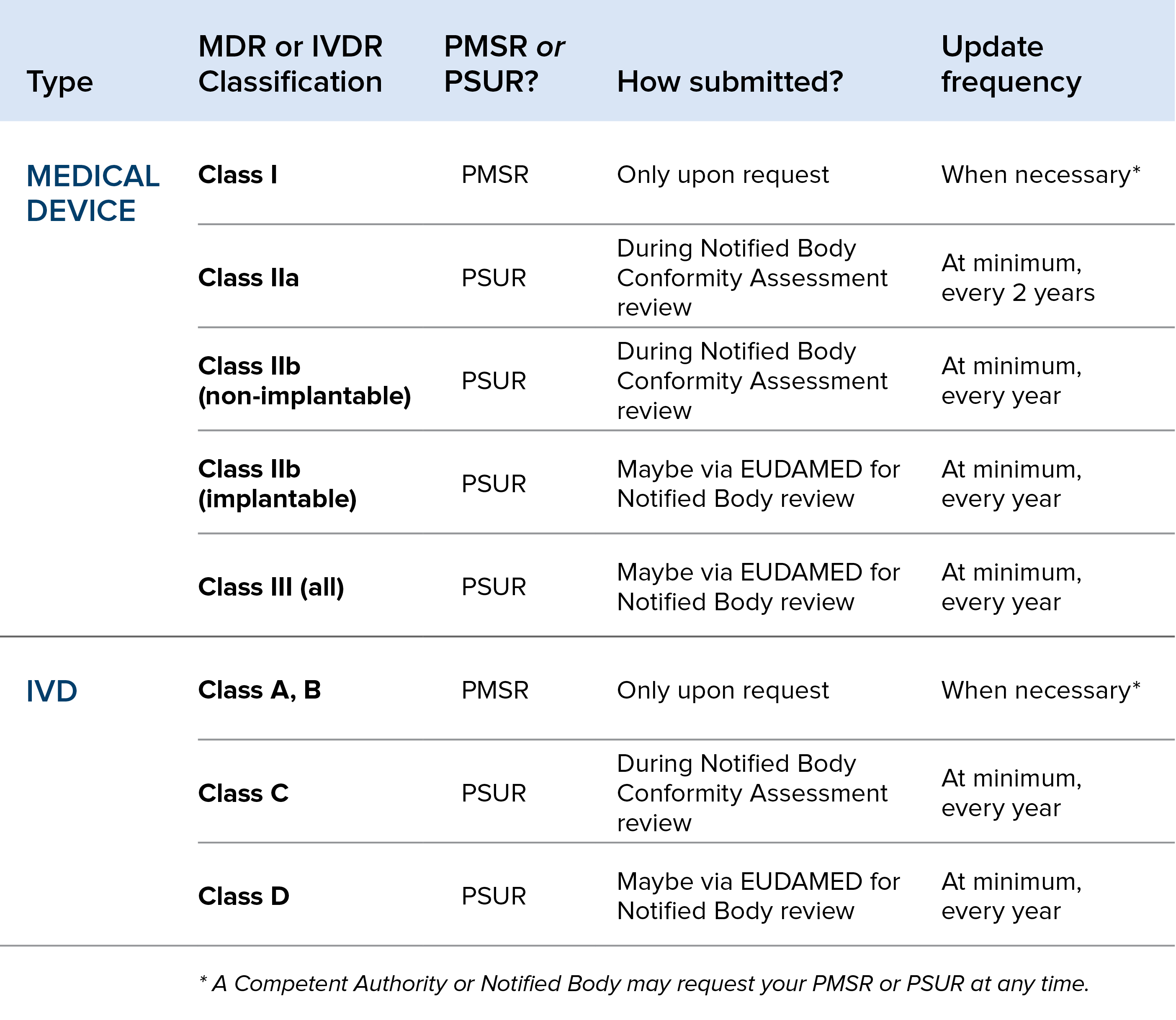
PMSR_PSUR Oriel STAT A MATRIX Blog

D75112 Periodic Safety Update Report Template Vee Care Asia
Structure Of periodic safety update report Pharmacovigilance

Psur PPT PSURs How They Should Be Used PowerPoint / What is

PSUR Requirements

PSUR Explained Everything You Need to Know About Periodic Safety

Periodic Safety Update Report Template Casus Consulting

Psur, Periodic Safety Update Report (PSUR), • how do i work out when to

Periodic Survey Update Template by Pharmi Med Ltd Issuu
Periodic Safety Update Report Contains All.
Cover Page Of Periodic Safety Update Report (Psur) (Rev 1).
Web This Page Includes Information On Periodic Safety Update Reports (Psurs), Psur Submission Requirements, Psur Single Assessment Procedures (Psusas) And The.
Web A Psur Is Generally Not Intended For The Notification Of Urgent New Safety Or Efficacy Information, Which May Have An Important Public Health Impact.
Related Post:
