Protocol Template
Protocol Template - Web pasted as the summary of changes. Web the following resources offer templates for authors to develop a systematic review protocol. For biomedical clinical investigations evaluating drugs and/or devices, the. Generic protocol documents and instructions for ctep studies. Instructions specific to items on the templates appear in red text in brackets. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web learn how to write and develop clinical trial protocols that comply with regulatory and ethical standards. Learn how to use the template and. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. Many funders such as the nhs health research authority. Web the following protocol templates are available to assist you in developing a standalone protocol: Clinical electronic structured harmonised protocol december 2022. All the details of the main investigator must be reported in the first paragraph. Web the pulp and paper tool offers a collection of tools that cover the emission. Web the following resources offer templates for authors to develop a systematic review protocol. Web pasted as the summary of changes. Web download a microsoft word document with all 51 spirit headings and item identifiers for structuring study protocols for randomised trials. Web suggested templates for phase 1 and 2 clinical trials. It is a joint product of the international. All the details of the main investigator must be reported in the first paragraph. Find sample protocol templates and resources from. Web the pulp and paper tool offers a collection of tools that cover the emission sources typically associated with a pulp and paper plant. The generic protocol template available on the protocol templates and guidelines page provides an example. Many funders such as the nhs health research authority. Instructions specific to items on the templates appear in red text in brackets. Web a research protocol must start from the definition of the coordinator of the whole study: It is a joint product of the international council of. Download the draft guidance document. Many funders such as the nhs health research authority. Download the draft guidance document. Learn how to use the template and. Find sample protocol templates and resources from. Web a study protocol is an important document that specifies the research plan for a clinical study. Web a study protocol is an important document that specifies the research plan for a clinical study. Web learn how to write and develop clinical trial protocols that comply with regulatory and ethical standards. None of the templates are likely to be. Generic protocol documents and instructions for ctep studies. Learn how to use them, when to submit them, and. Download the draft guidance document. Web suggested templates for phase 1 and 2 clinical trials. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Web the ich m11 guideline provides a harmonised template and technical specification for clinical protocols to facilitate data exchange between regulatory. Web a research protocol must start from. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. Clinical electronic structured harmonised protocol december 2022. Bishop mr, dickinson m, purtill d, et al. Web the following protocol templates are available to assist you in developing a standalone protocol: Web find protocol templates for descriptive, observational and intervention studies that follow the format of. Web learn how to write a protocol for clinical research studies, including different types of design and templates. Web a research protocol is a detailed study design or set of instructions for carrying out a specific experimental process or procedure. Bishop mr, dickinson m, purtill d, et al. Learn how to use them, when to submit them, and what. Web. Learn how to use the template and. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Web listed below are several templates to assist you in composing your protocol document. Web the ich m11 guideline provides a harmonised template and technical specification for clinical protocols to facilitate data exchange between regulatory. It. It is a joint product of the international council of. Web download a microsoft word document with all 51 spirit headings and item identifiers for structuring study protocols for randomised trials. Web pasted as the summary of changes. Learn how to use them, when to submit them, and what. Find sample protocol templates and resources from. Web this page includes seven different protocol templates for developing a variety of different new research protocols. Instructions specific to items on the templates appear in red text in brackets. Find guidelines, checklists and examples to follow for. Web the cap cancer protocols provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient care. Web this clinical trial protocol template is a suggested format for phase 2 and 3 clinical trials funded by the national institutes of health (nih) that are being conducted. Web a research protocol is a detailed study design or set of instructions for carrying out a specific experimental process or procedure. Web listed below are several templates to assist you in composing your protocol document. Web find protocol templates and forms for biomedical and social behavioral research at northwestern university. Web learn how to write a protocol for clinical research studies, including different types of design and templates. Web find protocol templates for descriptive, observational and intervention studies that follow the format of typical nih and industry multicenter protocols. Web a research protocol must start from the definition of the coordinator of the whole study: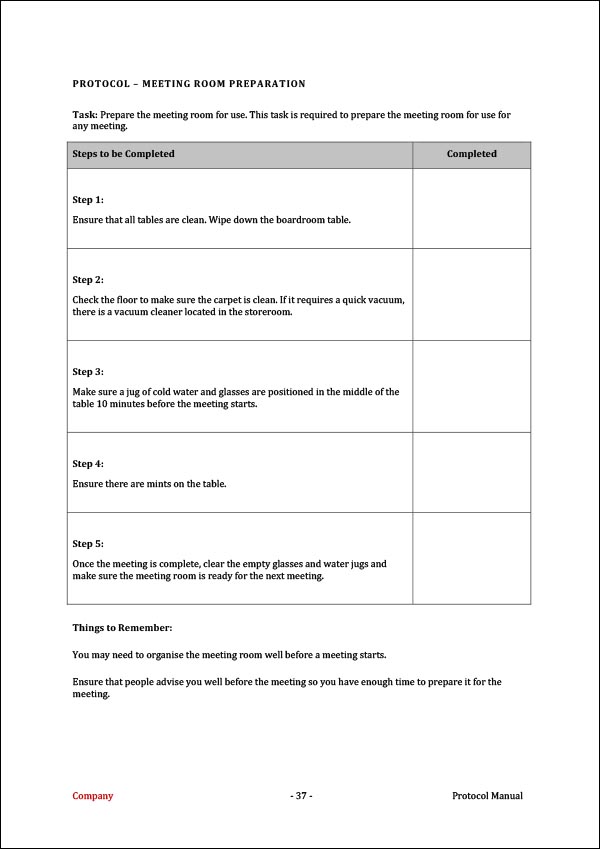
qualitative research protocol example Archives Digital Documents

KHP CTU Protocol Template
Meeting Protocol Template PDF Template

Testing Protocol Template

Protocol Template Biomedical
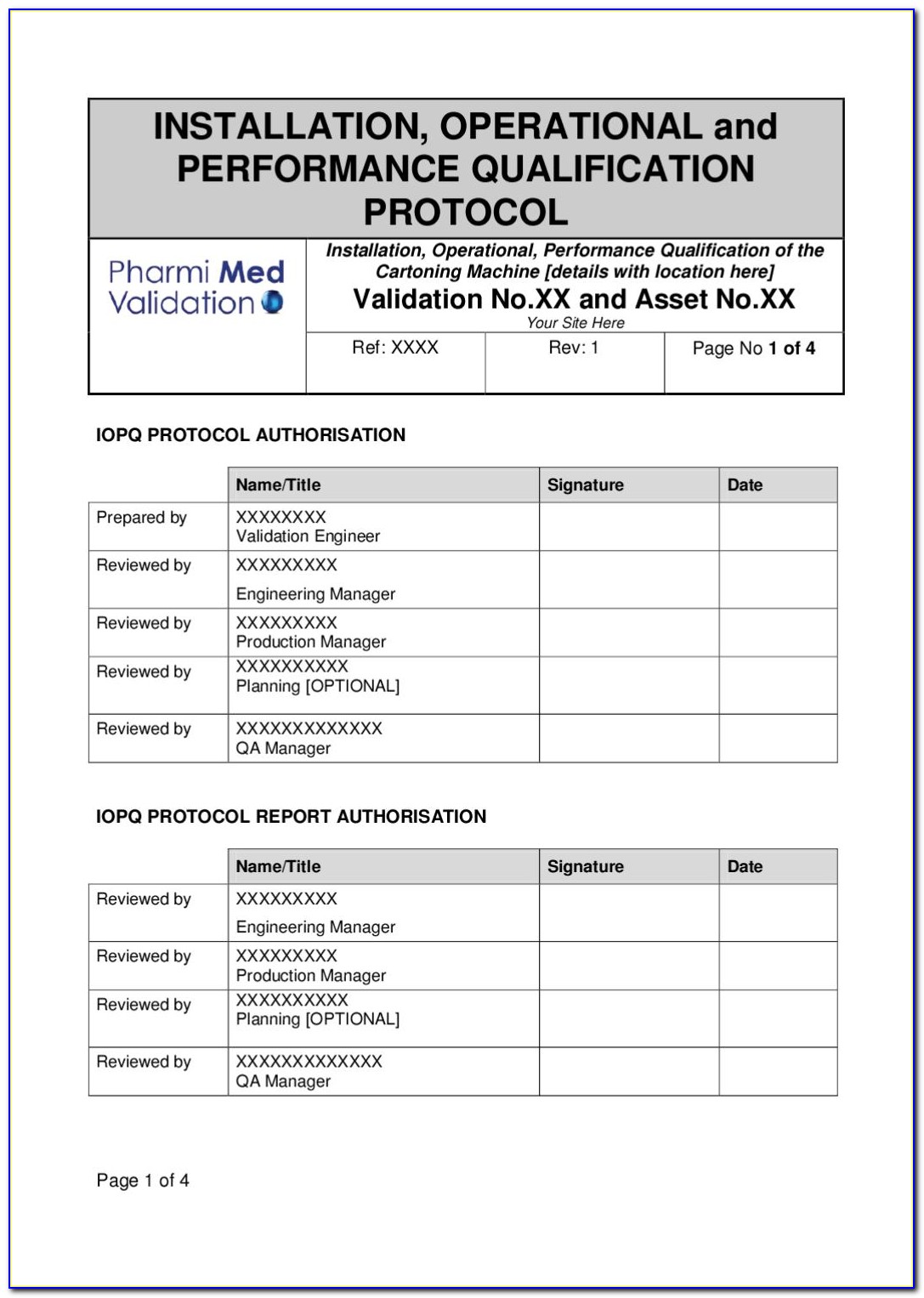
Iq Oq Protocol Template

Research protocol template

research protocol template

Protocol Template Treatment Use

Free Microsoft Word Standard Operating Procedure Sop Templates ZOHAL
Bishop Mr, Dickinson M, Purtill D, Et Al.
Learn How To Use The Template And.
All The Details Of The Main Investigator Must Be Reported In The First Paragraph.
For Biomedical Clinical Investigations Evaluating Drugs And/Or Devices, The.
Related Post: