Post Market Surveillance Plan Template
Post Market Surveillance Plan Template - Response to adverse events, when they occur , is. The mdr replaced the medical device directive (mdd) in may 2021. Once created, they are linked to your product record and show the. The template outlines the content, process and. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical. Web by regulatory & more oct 6, 2020. Describes the implementation of the pms system for collecting information and characterizing the safety and. Web a manufacturer must submit a postmarket surveillance plan within 30 calendar days of receipt of the 522 order.11 per section 522(b)(1) of the fd&c act and 21 cfr 822.17,. Web the templates outline the steps needed to create an effective and comprehensive plan. Manner that is proportionate to the risk. Web the templates outline the steps needed to create an effective and comprehensive plan. The template outlines the content, process and. Manner that is proportionate to the risk. It ensures that new information about safety and performance is proactively collected and. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to. Web the templates outline the steps needed to create an effective and comprehensive plan. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical. It ensures that new information about safety and performance is proactively collected and. Web check out this. Response to adverse events, when they occur , is. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical. 175 lines (134 loc) · 16.4 kb. Web check out this guidance document which may further help you to fill out the. The template outlines the content, process and. Manner that is proportionate to the risk. 175 lines (134 loc) · 16.4 kb. Once created, they are linked to your product record and show the. Describes the implementation of the pms system for collecting information and characterizing the safety and. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical. The mdr replaced the medical device directive (mdd) in may 2021. 175 lines (134 loc) · 16.4 kb. Describes the implementation of the pms system for collecting information and characterizing the. The template outlines the content, process and. Once created, they are linked to your product record and show the. The mdr replaced the medical device directive (mdd) in may 2021. Web the templates outline the steps needed to create an effective and comprehensive plan. It ensures that new information about safety and performance is proactively collected and. 175 lines (134 loc) · 16.4 kb. The mdr replaced the medical device directive (mdd) in may 2021. Once created, they are linked to your product record and show the. Web the templates outline the steps needed to create an effective and comprehensive plan. Web a manufacturer must submit a postmarket surveillance plan within 30 calendar days of receipt of. Web the templates outline the steps needed to create an effective and comprehensive plan. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously monitor the safety and the performance of the medical. Web by regulatory & more oct 6, 2020. The mdr replaced the medical device directive (mdd) in. Response to adverse events, when they occur , is. The mdr replaced the medical device directive (mdd) in may 2021. Manner that is proportionate to the risk. It ensures that new information about safety and performance is proactively collected and. Web a post market surveillance plan (pms plan) is a systematic plan of the processes and the activities to continuously. Web a manufacturer must submit a postmarket surveillance plan within 30 calendar days of receipt of the 522 order.11 per section 522(b)(1) of the fd&c act and 21 cfr 822.17,. Manner that is proportionate to the risk. Web by regulatory & more oct 6, 2020. Describes the implementation of the pms system for collecting information and characterizing the safety and.. Web check out this guidance document which may further help you to fill out the template: Response to adverse events, when they occur , is. Once created, they are linked to your product record and show the. 175 lines (134 loc) · 16.4 kb. The mdr replaced the medical device directive (mdd) in may 2021. It ensures that new information about safety and performance is proactively collected and. Web a manufacturer must submit a postmarket surveillance plan within 30 calendar days of receipt of the 522 order.11 per section 522(b)(1) of the fd&c act and 21 cfr 822.17,. The template outlines the content, process and. Web by regulatory & more oct 6, 2020. Describes the implementation of the pms system for collecting information and characterizing the safety and.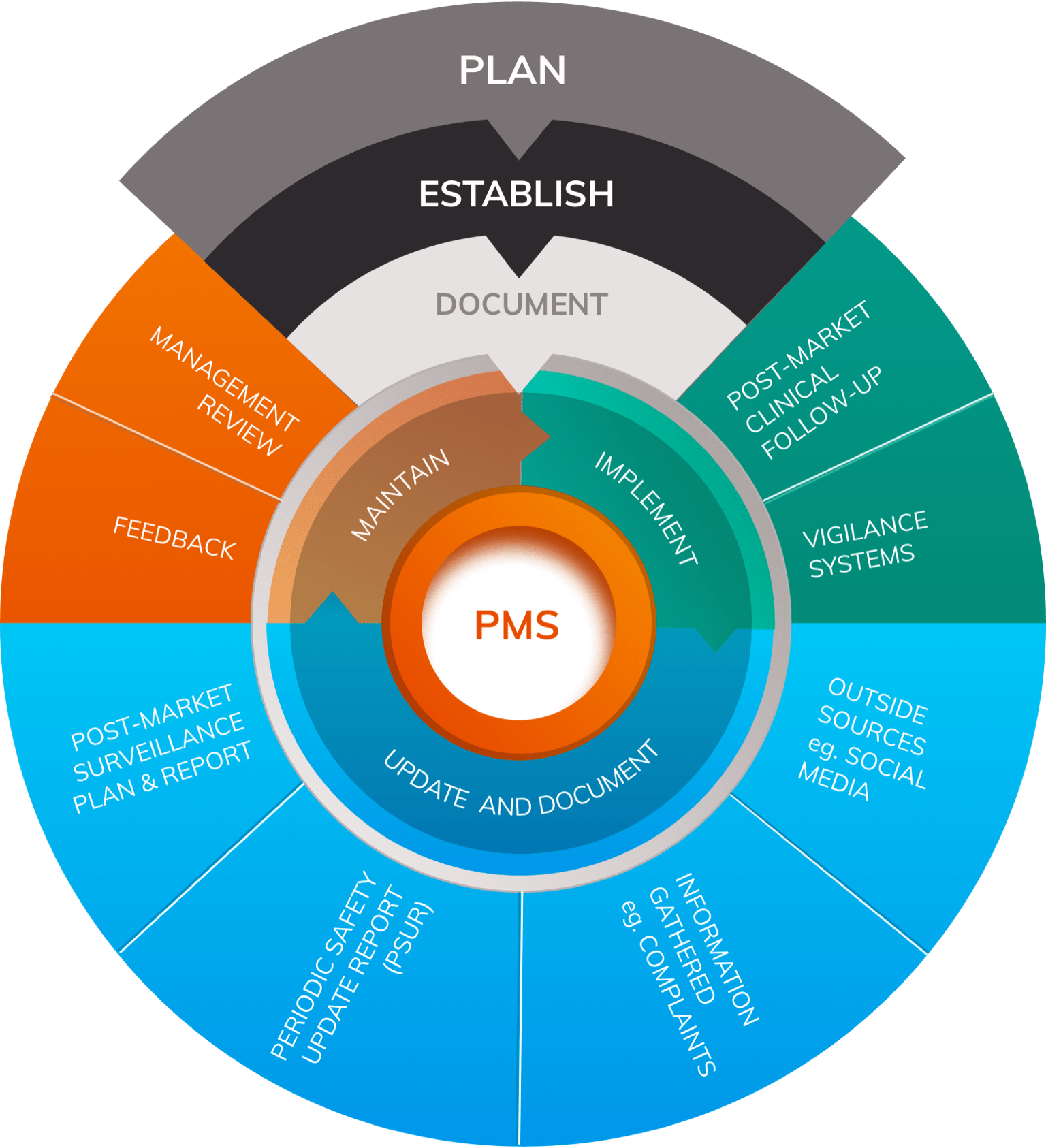
PostMarket Surveillance (PMS) of medical devices

Postmarket surveillance plans How to write one for CE Marking.
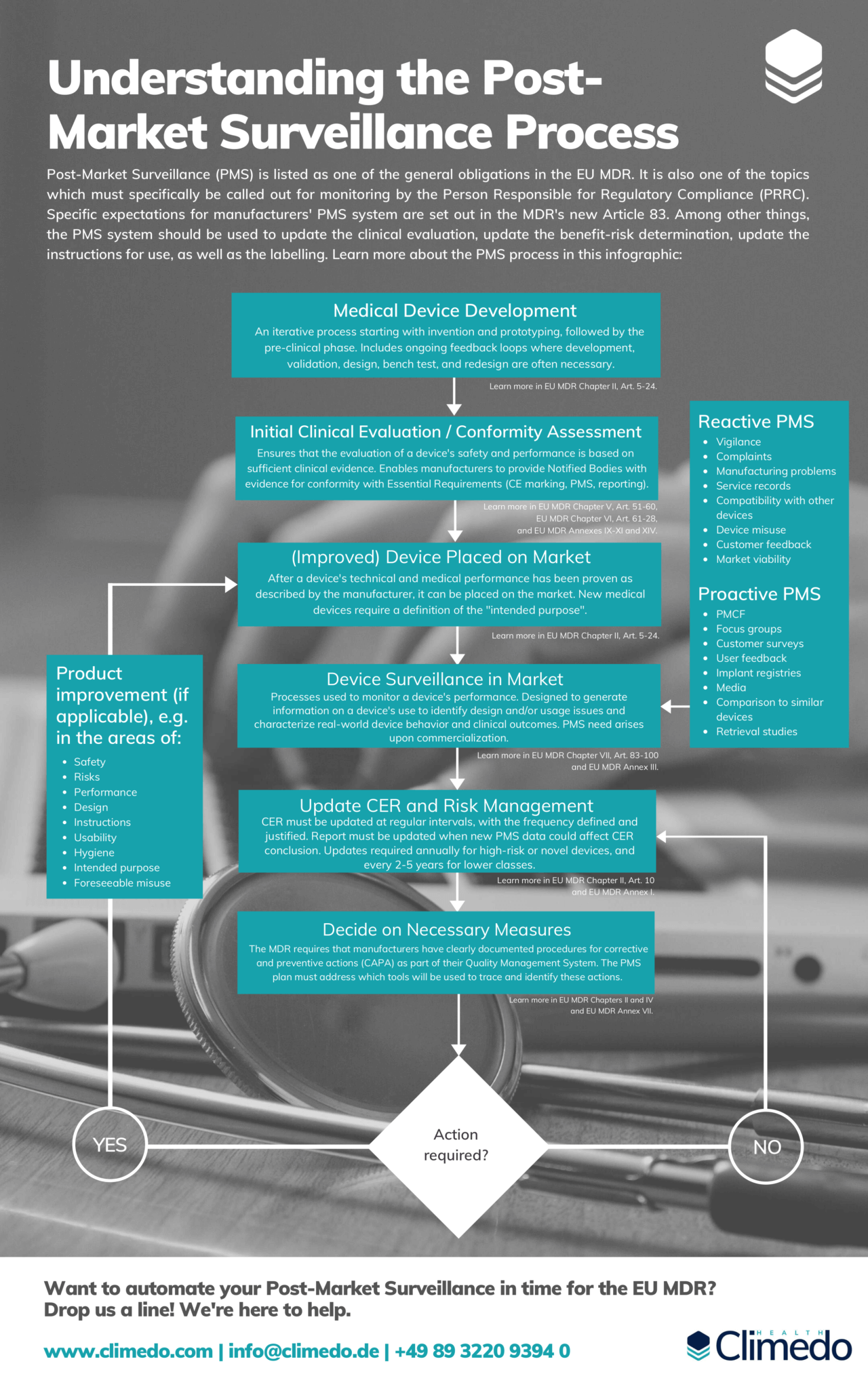
Getting your PostMarket Surveillance up to Speed with the EU MDR
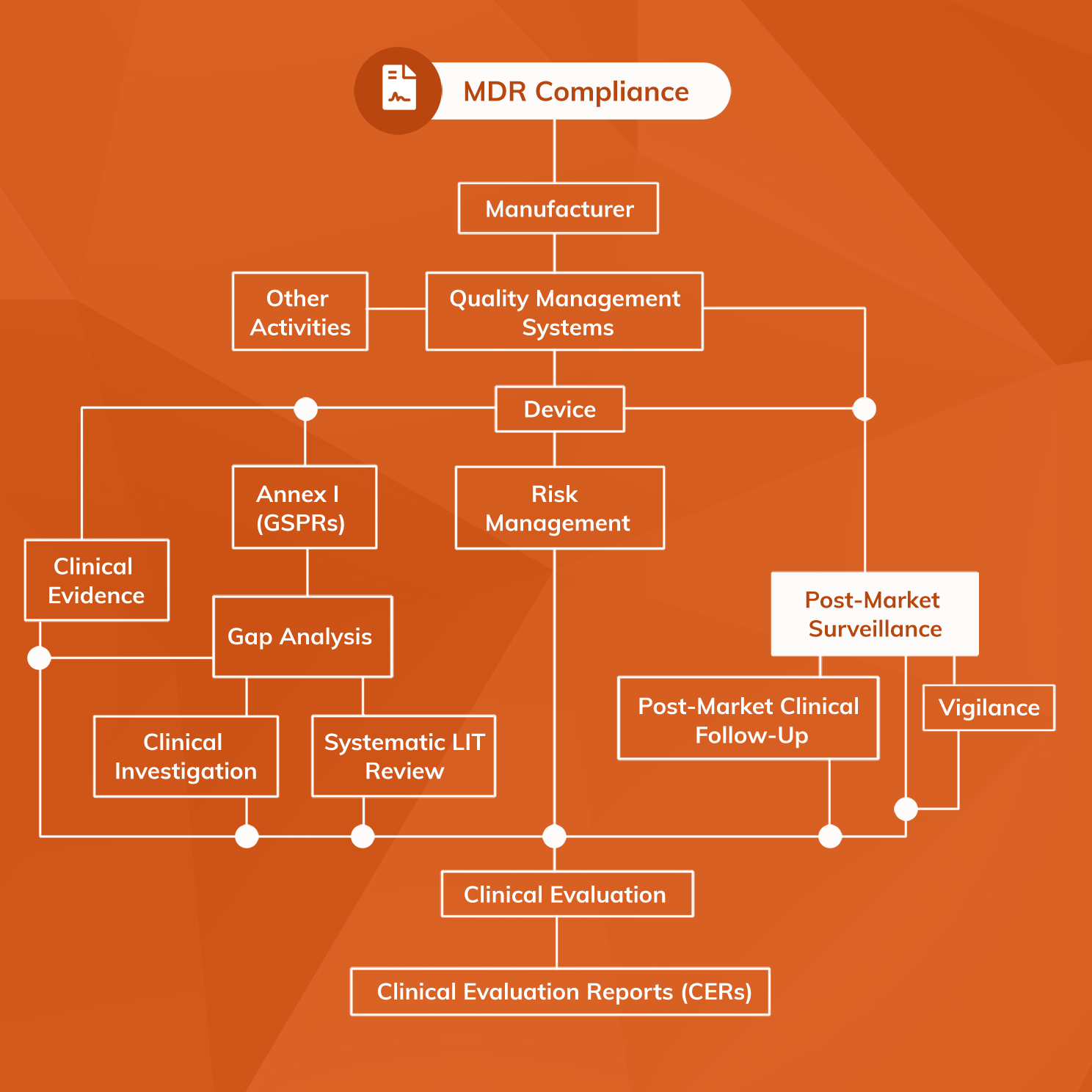
PostMarket Surveillance (PMS) of medical devices
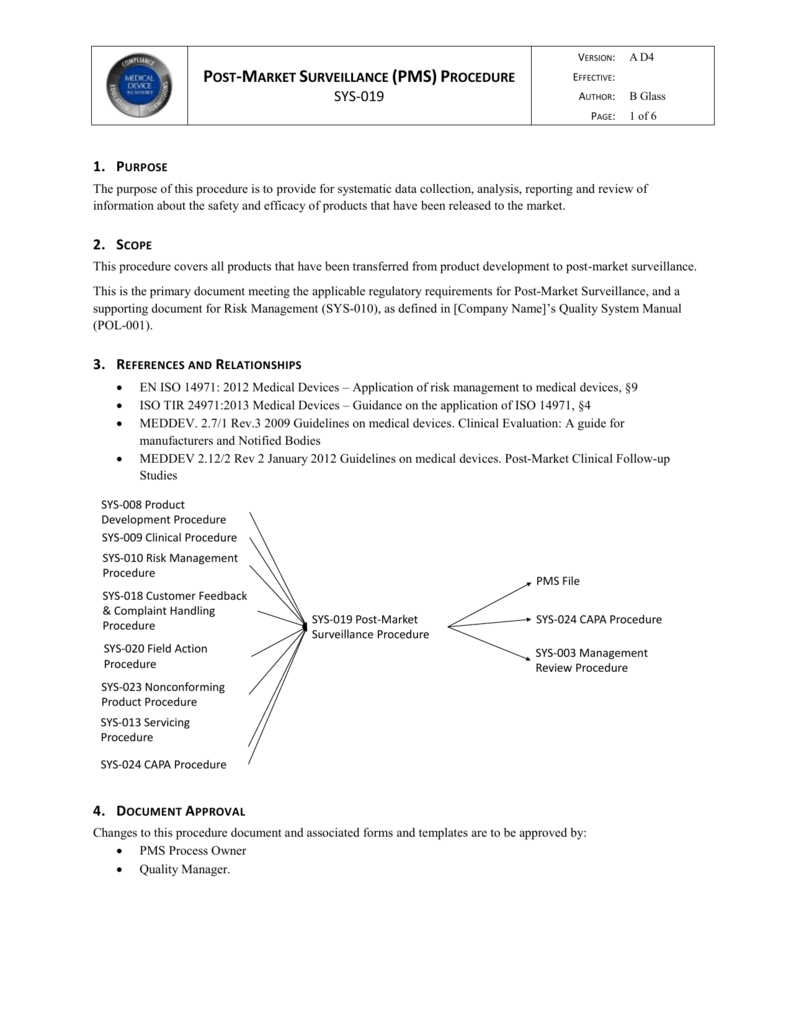
Postmarket surveillance is in itself a monitoring and measuring

MDR/IVDR Postmarket Surveillance 2023 Starter Guide Casus

PostMarket Surveillance Plan Template QualityMedDev
Output of the postmarket surveillance (PMS) plan [Colour figure can be

Post Market Surveillance Plan PMS Plan Template

PostMarket Surveillance Plan Template QualityMedDev
Manner That Is Proportionate To The Risk.
Web The Templates Outline The Steps Needed To Create An Effective And Comprehensive Plan.
Web A Post Market Surveillance Plan (Pms Plan) Is A Systematic Plan Of The Processes And The Activities To Continuously Monitor The Safety And The Performance Of The Medical.
Related Post: