Patterns In Electron Configurations Answer Key
Patterns In Electron Configurations Answer Key - Web ns f4 electron configuration answers ground state orbital diagrams and electron configurations compare the ground state orbital diagrams below to the boarding house diagrams on page 1. Answer co has 27 protons, 27 electrons, and 33 neutrons: 1s2 2s2 draw the electron configurations of the following atoms, [kr]5s24d105p6 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. Electron configurations and periodic trends. Identify the atom represented below. Exend (30 minutes) summary students will use the atoms sandbox to continue to practice the concept of electron configuration and fill order. Teacher notes names key class date. Follow the instructions to go through the simulation. It just tells you where the electrons are. Web examine patterns in the electron configurations of various ions and atoms. Note that there is a pattern to electron configurations. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web write the complete electron configuration for each isotope. Web electron configuration atomic radius (pm) aluminum 13 1s^2, 2s^2, 2p^6, 3s^2, 3p^1 118 silicon. [kr]5s24d105p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5 iodine anion: Click next element, and then add an electron to the magnesium atom.click check, and record the electron configuration. Web ns f4 electron configuration answers ground state orbital diagrams and electron configurations compare the ground state orbital diagrams below to the boarding house diagrams on page. 1s2 2s2 draw the electron configurations of the following atoms, Examine the orbital diagrams and electron configurations as shown. Electron configurations and periodic trends. Exend (30 minutes) summary students will use the atoms sandbox to continue to practice the concept of electron configuration and fill order. This video worksheet will help you understand the pattern. 21) 1s22s22p63s1 sodium 22) 1s22s22p63s23p64s23d104p65s24d6 ruthenium 23) [kr] 5s24d10 cadmium 24) [xe] 6s24f145d106p2 lead 25) [rn] 7s25f146d4 seaborgium determine if the following electron configurations are correct: Answer co has 27 protons, 27 electrons, and 33 neutrons: Download the naming electron configuration worksheet. It just tells you where the electrons are. Teacher notes names key class date. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. Web electron configurations are a simple way of writing down the locations of all of the electrons in an atom. There is a simple pattern that you will see in a few minutes by using the below examples. Write the ground state electron configuration for. The electron configurations and orbital diagrams of these four elements are: Teacher notes names key class date. Create a proper electron configuration for sodium. Web electrons are distributed in the electron cloud into principal energy levels (1 , 2,3 ) sublevels (s, p, d, f), orbitals (s has i , p has 3, d has 5, f has 7) and. Compare the electron configurations below to the manger’s code, also on page 1. Introduction one of the many patterns contained in the periodic table is that of electron configuration. Web electron configuration atomic radius (pm) aluminum 13 1s^2, 2s^2, 2p^6, 3s^2, 3p^1 118 silicon 14 1s^2, 2s^2, 2p^6, 3s^2, 3p^2 111 phosphorus 15 1s^2, 2s^2, 2p^6, 3s^2, 3p^3 98 sulfur. Web the patterns in electron configuration answer key follows: Answer co has 27 protons, 27 electrons, and 33 neutrons: After clicking check, note the electron configuration and the atomic radius now listed at right.; The alkali metal sodium (atomic number 11) has one more electron than the neon atom. It just tells you where the electrons are. In this activity, you will identify these patterns. Draw the electron configuration of sodium (atomic #11). Follow the instructions to go through the simulation. [kr]5s24d105p6 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. 21) 1s22s22p63s1 sodium 22) 1s22s22p63s23p64s23d104p65s24d6 ruthenium 23) [kr] 5s24d10 cadmium 24) [xe] 6s24f145d106p2 lead 25) [rn] 7s25f146d4 seaborgium determine if the following electron. Web electron configuration atomic radius (pm) aluminum 13 1s^2, 2s^2, 2p^6, 3s^2, 3p^1 118 silicon 14 1s^2, 2s^2, 2p^6, 3s^2, 3p^2 111 phosphorus 15 1s^2, 2s^2, 2p^6, 3s^2, 3p^3 98 sulfur 16 1s^2, 2s^2, 2p^6, 3s^2, 3p^4 88 chlorine 17 1s^2, 2s^2, 2p^6, 3s^2, 3p^5 79 argon 18 1s^2, 2s^2, 2p^6, 3s^2, 3p^6 71 Understand the arrangement of electrons within. Write the electron configuration of arsenic (as) in long notation and in short (noble gas) notation. It just tells you where the electrons are. Write the ground state electron configuration for neutral hydrogen and then write the electron configuration for an excited state of hydrogen. After clicking check, note the electron configuration and the atomic radius now listed at right.; 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3 [ar] 4s2 3d10 4p3. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Web find answers to all your questions about electron configurations with the electron configuration gizmo. Web an electron configuration is a list that shows you where all of the electrons in an atom are located. Compare the electron configurations below to the manger’s code, also on page 1. [kr]5s24d105p6 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. Web use the patterns within the periodic table to write the shorthand electron configurations for the following elements. Web ns f4 electron configuration answers ground state orbital diagrams and electron configurations compare the ground state orbital diagrams below to the boarding house diagrams on page 1. There’s an answer key too in the other pdf file. Identify the atom represented below. Web the following electron configurations belong to which elements: 21) 1s22s22p63s1 sodium 22) 1s22s22p63s23p64s23d104p65s24d6 ruthenium 23) [kr] 5s24d10 cadmium 24) [xe] 6s24f145d106p2 lead 25) [rn] 7s25f146d4 seaborgium determine if the following electron configurations are correct:
Patterns in Electron Configuration

Patterns In Electron Configuration Worksheet Answers
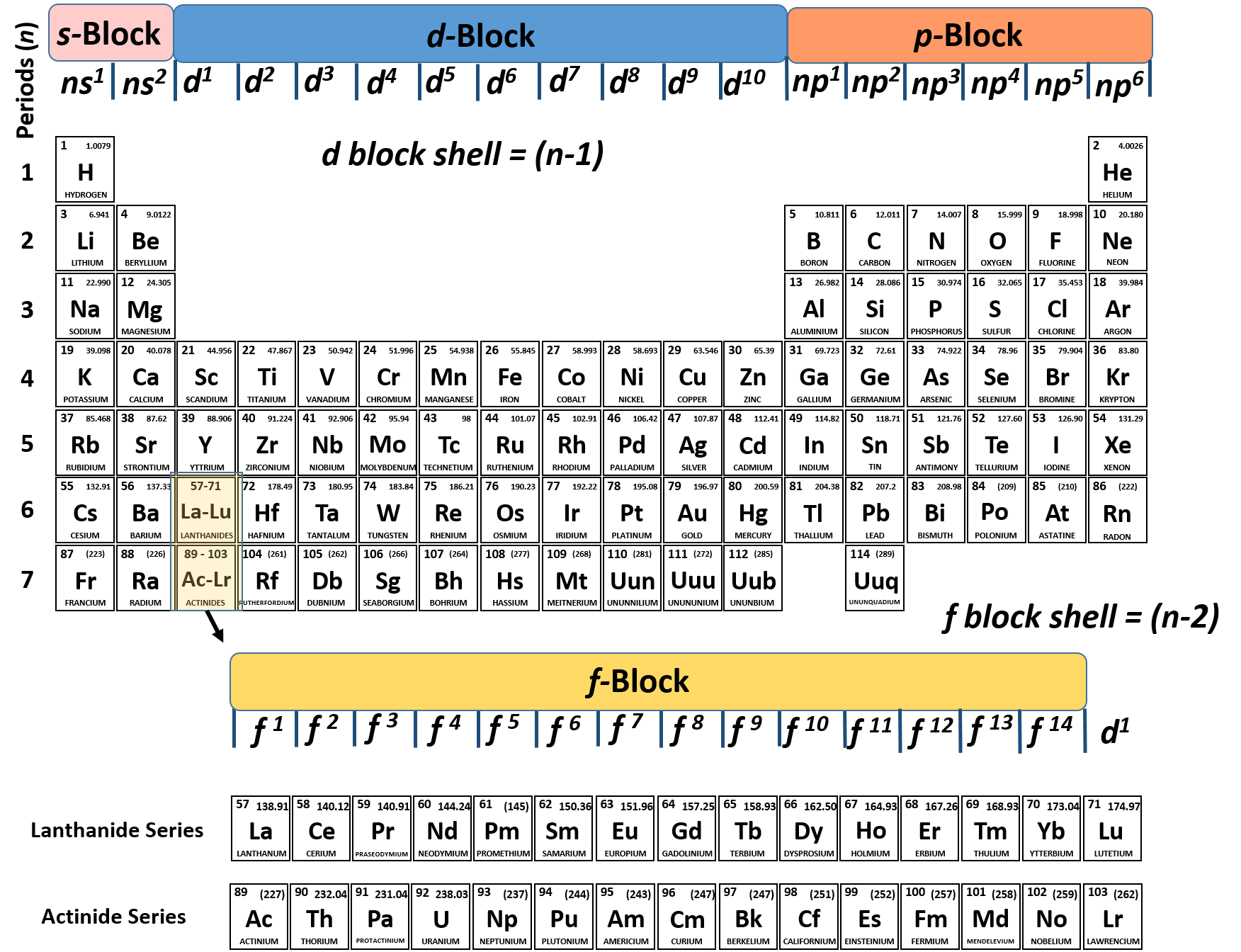
CH104 Chapter 2 Atoms and The Periodic Table Chemistry
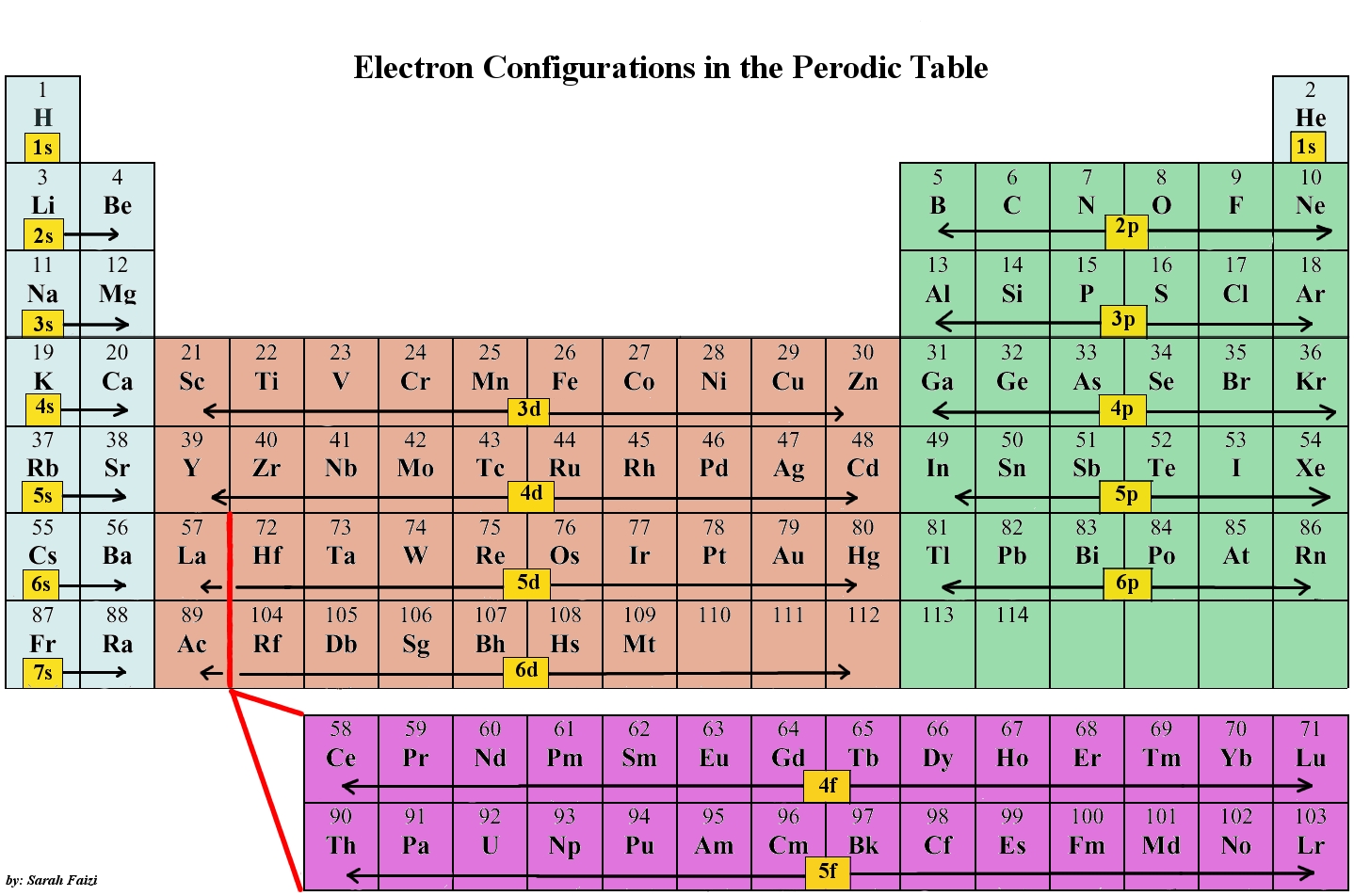
Electronic Configurations Chemwiki

Patterns in electron configuration YouTube

Electron Configuration Worksheet Easy Hard Science

Patterns In Electron Configuration Worksheet Answers

List of Electron Configurations of Elements

Patterns in Electron Configurations YouTube
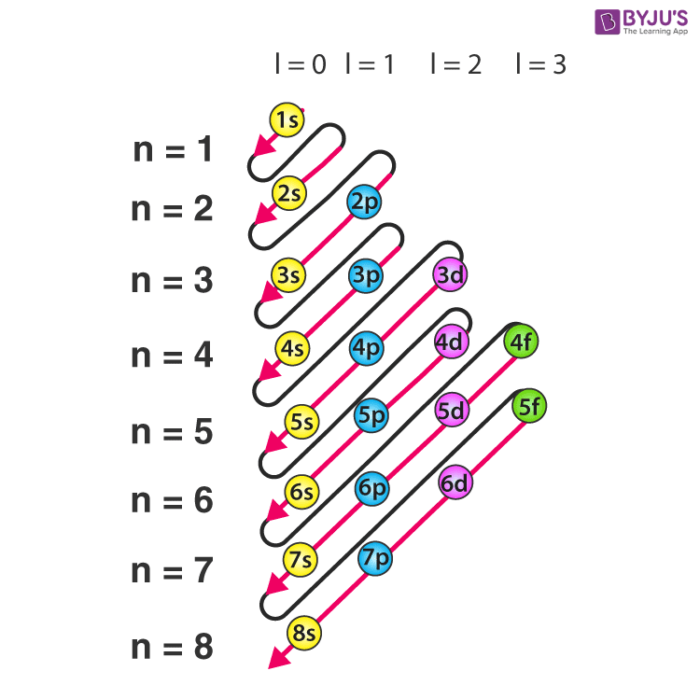
Electronic configuration detailed explanation, orbital filling
This Video Worksheet Will Help You Understand The Pattern.
The Electron Configurations In This Worksheet Assume That Lanthanum (La) Is The First Element In The 4F Block And That Actinium (Ac) Is The First Element In The 5F Block.
Note That There Is A Pattern To Electron Configurations.
Web The Patterns In Electron Configuration Answer Key Follows:
Related Post: