Paediatric Investigation Plan Template
Paediatric Investigation Plan Template - Web 1) define the pip strategy early in the writing process. List of required documents by procedure type. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. Web paediatric investigation plan (pip). Outline of paediatric submission steps. This page provides detailed guidance for companies intending to apply for a paediatric investigation plan (pip),. Web table of contents 1. 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans. Web guideline on the format and content of applications for agreement or modification of a paediatric investigation plan and requests for waivers or deferrals. Outline of paediatric submission steps. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. Web this page lists the templates and forms required by companies wishing to apply for a paediatric investigation plan (pip), deferral or waiver. 2023, the european medicines agency (ema) issued new european union (eu). This page provides detailed guidance for companies intending to apply for a paediatric investigation plan (pip),. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project. Outline of paediatric submission steps. Web guideline on the format and content of. List of required documents by procedure type. List of required documents by procedure type. Web paediatric investigation plan (pip). Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template ’) and the. A paediatric investigation plan (pip) is a development plan aimed at ensuring. Application for a paediatric investigation plan or waiver author: Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project. List of required documents by procedure type. Outline of paediatric submission steps. Web foremost among these are the electronic form. Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template ’) and the. Legal requirements for children's medicines Web a pediatric investigation plan (pip, required in the european union) or pediatric study plan (psp, required in the united states) is a development plan aimed. Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template ’) and the. Web 1) define the pip strategy early in the writing process. Legal requirements for children's medicines Outline of paediatric submission steps. Web the main challenges for medical writers when writing a. Legal requirements for children's medicines It is important to carefully consider the most relevant condition and indication for your product in the entire. Web a pediatric investigation plan (pip, required in the european union) or pediatric study plan (psp, required in the united states) is a development plan aimed at ensuring that. Web this page lists the templates and forms. Outline of paediatric submission steps. A paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, when. Outline of paediatric submission steps. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. List of required documents by procedure type. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project. Application for a paediatric investigation plan or waiver. Web table of contents 1. 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans. Outline of paediatric submission steps. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input. This common commentary addresses only the submission of an ipsp and pip for a drug or biological product for treatment or. 2023, the european medicines agency (ema) issued new european union (eu) guidance for drug developers regarding the conduct of paediatric investigation plans. Outline of paediatric submission steps. Web format and content of applications for agreement or modification of a paediatric investigation plan; Center for drug evaluation and research center for biologics evaluation and research the purpose of this guidance is to provide recommendations to. Web 1) define the pip strategy early in the writing process. European medicines agency created date: Outline of paediatric submission steps. Content of and process for submitting initial pediatric study plans and amended initial pediatric study plans guidance for industry u.s. Web foremost among these are the electronic form for paediatric investigation plan application and request for waiver (a pdf file sometimes referred to as the‘pip template ’) and the. It is important to carefully consider the most relevant condition and indication for your product in the entire. This page provides detailed guidance for companies intending to apply for a paediatric investigation plan (pip),. Legal requirements for children's medicines List of required documents by procedure type. A paediatric investigation plan (pip) is a development plan aimed at ensuring that the necessary data are obtained through studies in children, when. Web the main challenges for medical writers when writing a pip are application of the guidance to the drug and disease in hand, and obtaining the appropriate input from the project.
Medicines for Children Trilogy Writing & Consulting

Planning your Paediatric Investigation Plan (PIP) Submission in Euro…

Fillable Online Paediatric investigation plans questions and answers
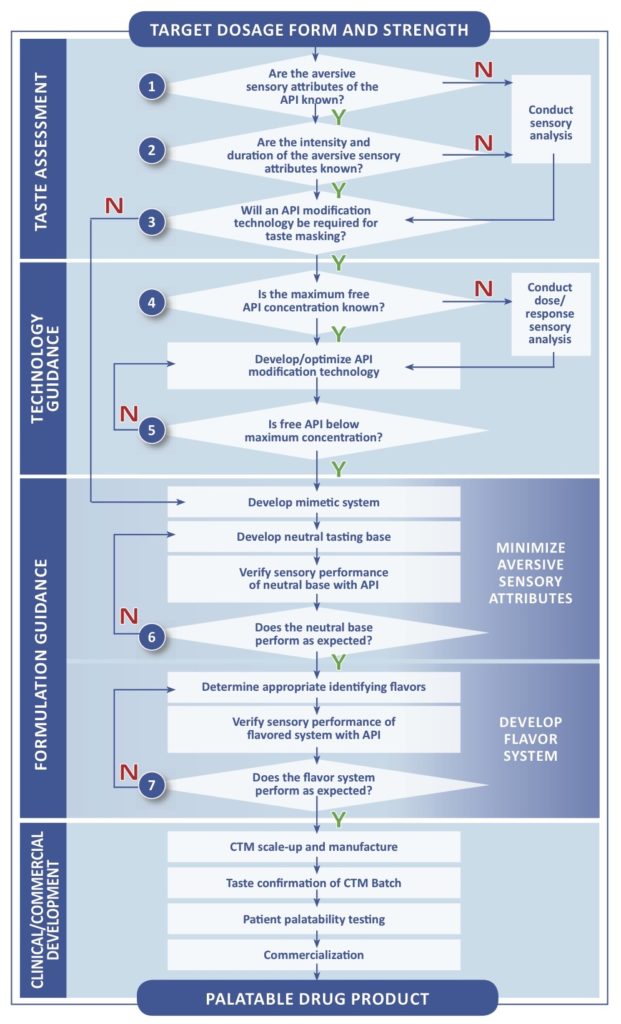
Pediatric Investigation Plans Part 1 Determining Taste Masking
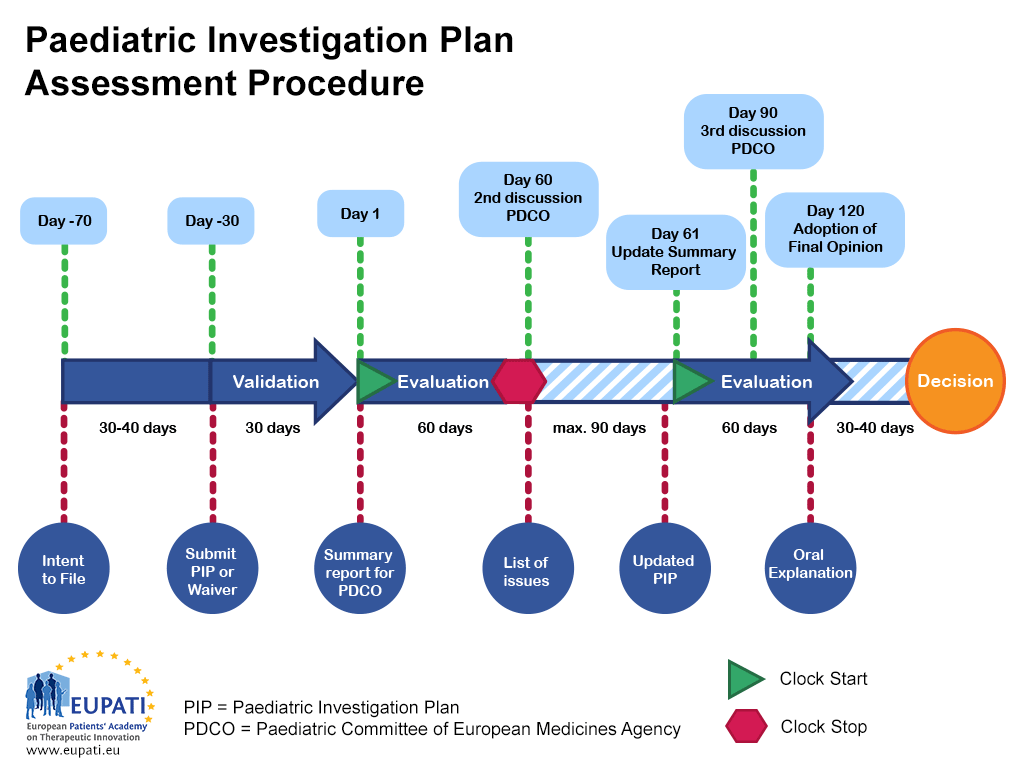
Paediatric medicine Paediatric Investigation Plan EUPATI Toolbox

Overview of current paediatric investigation plan (PIP) application

Planning your Paediatric Investigation Plan (PIP) Submission in Euro…

Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Pediatric Care Plan Template Medical Diagnosis Nursing

Planning your Paediatric Investigation Plan (PIP) Submission in Euro…
Web This Page Lists The Templates And Forms Required By Companies Wishing To Apply For A Paediatric Investigation Plan (Pip), Deferral Or Waiver.
List Of Required Documents By Procedure Type.
Web Guideline On The Format And Content Of Applications For Agreement Or Modification Of A Paediatric Investigation Plan And Requests For Waivers Or Deferrals.
Web A Pediatric Investigation Plan (Pip, Required In The European Union) Or Pediatric Study Plan (Psp, Required In The United States) Is A Development Plan Aimed At Ensuring That.
Related Post:
