Operational Qualification Performance Qualification Template
Operational Qualification Performance Qualification Template - Web installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and. The other two tests are operational qualification (oq) and. Download a sample executed executed operational. These are the abbreviations we use in the medical device industry for the three steps of process. Web the objective of the operations qualification is to perform a series of tests to determine that the operation of the equipment/process conforms to the specifications. Web download a sample operational qualification, based on the fastval operational qualification template. Web installation qualification (iq) is one of the first testing phases during an equipment or system qualification. 3 table 1 shows the most common. Sops for method validation and equipment testing. Web the dq protocol section of this qualification package defines and validates the freezer system design. The other two tests are operational qualification (oq) and. What is iq, oq, pq? 3 table 1 shows the most common. What is an operational qualification oq? Web installation qualification (iq) is one of the first testing phases during an equipment or system qualification. Web installation qualification (iq) is one of the first testing phases during an equipment or system qualification. For an example of protocol execution, see our fastval electronic protocol execution. A performance qualification template is used to complete the process validation protocol by detailing. Web for more examples, see our operational qualification template. Download a sample executed executed operational. This incorporates a range of testing to simulate your production process options. Web operational qualification operational qualification (oq) demonstrates that the experion automated electrophoresis station and software are functioning to specification. Web an operational qualification template is used to complete the process validation protocol by recording all required data such as calibration equipment, training records, and user’s. Web installation qualification. Help to improve quality and to comply with glp, gcp,. Web the objective of the operations qualification is to perform a series of tests to determine that the operation of the equipment/process conforms to the specifications. What is iq, oq, pq? Web the dq protocol section of this qualification package defines and validates the freezer system design. Things to consider…. These are the abbreviations we use in the medical device industry for the three steps of process. The other two tests are operational qualification (oq) and. Web the dq protocol section of this qualification package defines and validates the freezer system design. Web operational qualification (oq) performance qualification (pq) laboratory user requirements specification. What is an operational qualification oq? Web for more examples, see our operational qualification template. These are the abbreviations we use in the medical device industry for the three steps of process. Web installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and. A performance qualification template is used to complete the process validation protocol by detailing. 3. What is iq, oq, pq? This incorporates a range of testing to simulate your production process options. Web for more examples, see our operational qualification template. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name. The other two tests are operational qualification (oq). Web operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. Web the objective of the operations qualification is to perform a series of tests to determine that the operation of the equipment/process conforms to the specifications. The dq protocol section also defines and validates the manufacturer. Web performance qualification (pq) demonstrate the process will. Web installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument performance verification (ipv) services verify and. Web operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. The other two tests are operational qualification (oq) and. Web installation qualification (iq) is one of the first testing phases during an equipment or system. Web an operational qualification template is used to complete the process validation protocol by recording all required data such as calibration equipment, training records, and user’s. Help to improve quality and to comply with glp, gcp,. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert. Web the operational qualification protocol (checklist) template includes a detailed outline of all tests and procedures to be performed during the oq process. Web for more examples, see our operational qualification template. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name. Web the objective of the operations qualification is to perform a series of tests to determine that the operation of the equipment/process conforms to the specifications. These are the abbreviations we use in the medical device industry for the three steps of process. Things to consider… • approved. Web an operational qualification template is used to complete the process validation protocol by recording all required data such as calibration equipment, training records, and user’s. Web the dq protocol section of this qualification package defines and validates the freezer system design. Web the p1q represents the final performance qualification template of your equipment or system. The other two tests are operational qualification (oq) and. The dq protocol section also defines and validates the manufacturer. 3 table 1 shows the most common. Web operational qualification (oq) involves identifying and inspecting equipment features that can impact final product quality. The operational qualification is a series of tests to demonstrates if the process or. For an example of protocol execution, see our fastval electronic protocol execution. Web download a sample operational qualification, based on the fastval operational qualification template.
Operation Qualification & Installation Qualification Hamilton Company
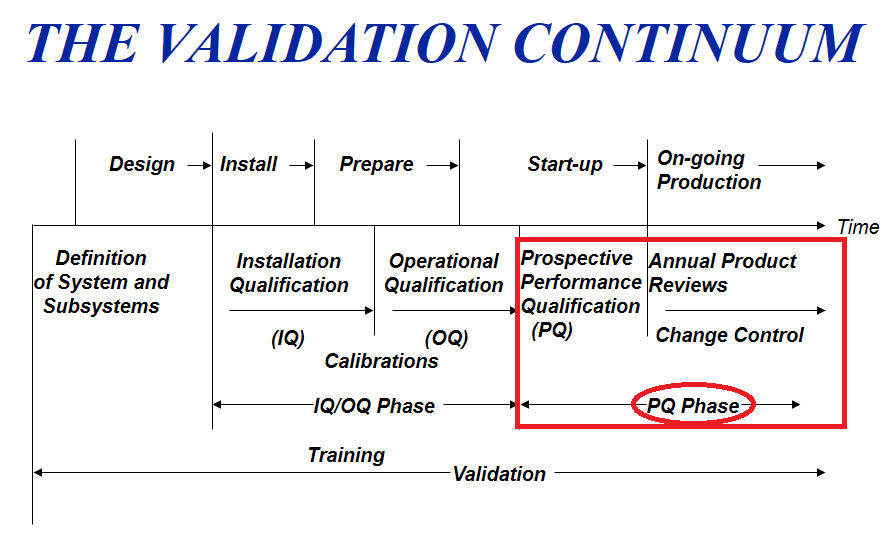
Performance Qualification

Performance Evaluation in Word and Pdf formats page 3 of 4

Six Sigma Validation Process IQ Installation Qualification OQ

22+ Statement of Qualifications Templates in PDF DOC
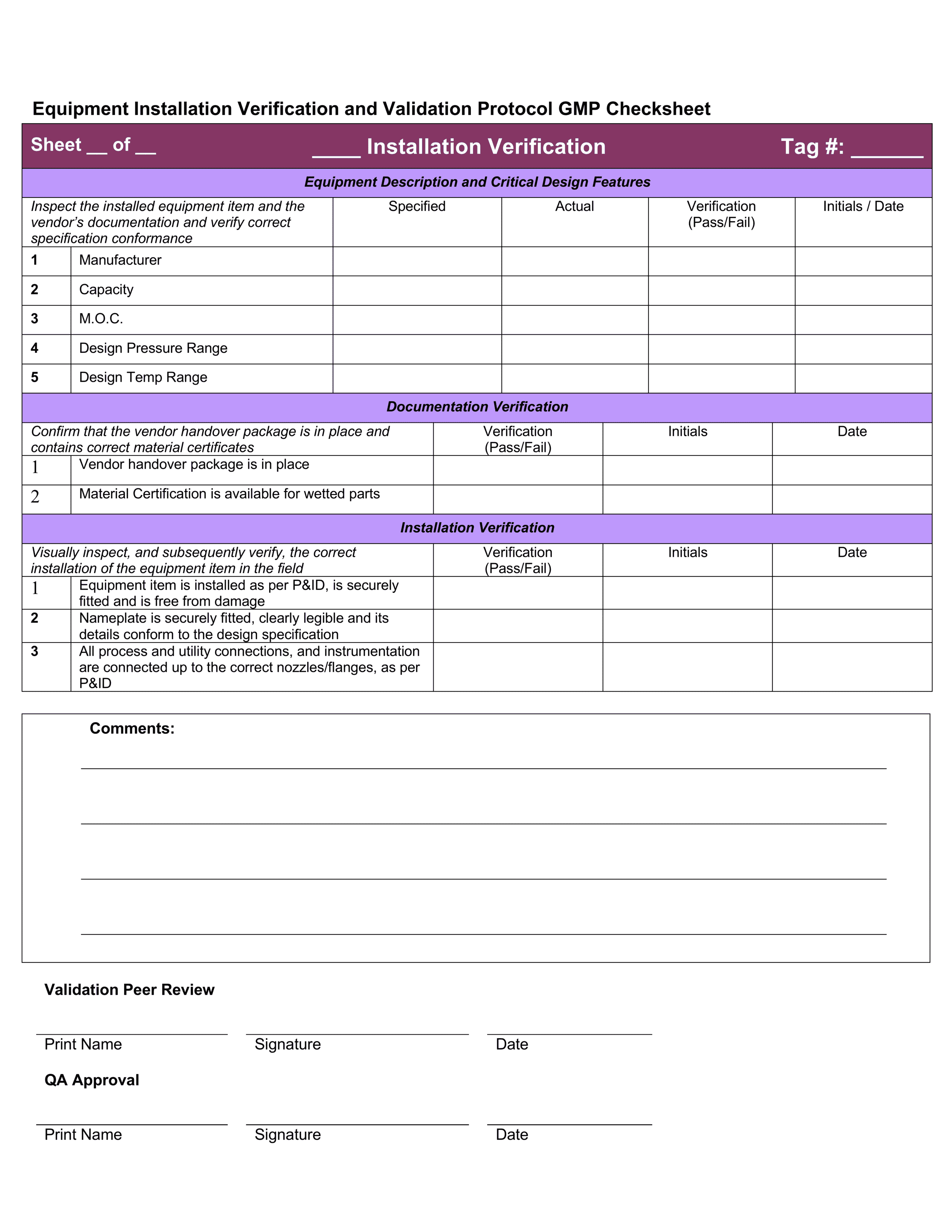
Free Iq Oq Pq Template Printable Form, Templates and Letter
CIQA Installation and Operational Qualification Protocol IOQ Equipment
Operational Qualification Template Verification And Validation

PPT 3. Validation (and Qualification) PowerPoint Presentation, free

Iq Oq Pq Template Doc Templates MjIyMTY Resume Examples
Web Operational Qualification (Oq) Performance Qualification (Pq) Laboratory User Requirements Specification.
What Is An Operational Qualification Oq?
Help To Improve Quality And To Comply With Glp, Gcp,.
Download A Sample Executed Executed Operational.
Related Post:

