O2 Drawing
O2 Drawing - Connect the atoms to each other with single bonds to form a “skeleton structure.”. In order to draw the lewis structure of o2, first of all you have to find the total number of valence electrons present in the o2 molecule. Use these steps to correctly draw the o 2 lewis structure: First of all, we calculate the total valence electrons for the o2 molecule. Web an arterial blood gas (abg) test is a blood test that requires a sample from an artery in your body to measure the levels of oxygen and carbon dioxide in your blood. The o 2 lewis structure indicates that the o 2 molecule is perfectly symmetric. So to attain stability according to the octet. Oxygen belongs to the 16 th group of the periodic table and has 6 valence electrons. It also is a good example of a molecule with a double bond. I draw the electron configuration diagram in this video.then, i s. (valence electrons are the electrons that are present in the outermost orbit of any atom.). Web the drawing of lewis structure of o2 helps us to find out the previous important part. Web the lewis dot structure of beryllium and oxygen is relatively simple. However those all steps are. Your body normally tightly regulates the amount of oxygen and carbon. Web this widget gets the lewis structure of chemical compounds. Generally, small symmetric molecules are nonpolar. The o 2 lewis structure indicates that the o 2 molecule is perfectly symmetric. Web drawing the lewis structure for o 2 ( dioxygen or oxygen gas) oxygen (o 2) is a commonly tested lewis structure due to it's importance on earth. Use these. Web in this video we'll look at the atomic structure and bohr model for the oxygen atom (o). Because o 2 is a simple molecule, it is extremely easy to draw the lewis structure of it. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. I draw the electron configuration. Web steps of drawing lewis diagram. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Valence electrons are the most important things to draw lewis structure. Web drawing the lewis structure for o. (valence electrons are the electrons that are present in the outermost orbit of any atom.). Web an arterial blood gas (abg) test is a blood test that requires a sample from an artery in your body to measure the levels of oxygen and carbon dioxide in your blood. In order to find the total valence electrons in o2 (oxygen) molecule, first of all you should know the valence electrons present in a single oxygen atom.. Web in this video we'll look at the atomic structure and bohr model for the oxygen atom (o). Web an arterial blood gas (abg) test is a blood test that requires a sample from an artery in your body to measure the levels of oxygen and carbon dioxide in your blood. So to attain stability according to the octet. Drawing. Web the lewis dot structure of beryllium and oxygen is relatively simple. In order to find the total valence electrons in o2 (oxygen) molecule, first of all you should know the valence electrons present in a single oxygen atom. That's the only way you can make it work with the twelve valence electrons available. Web an arterial blood gas (abg). There are 12 valence electrons available for the lewis structure for o 2. Web drawing the lewis structure for o 2 ( dioxygen or oxygen gas) oxygen (o 2) is a commonly tested lewis structure due to it's importance on earth. Some molecules must have multiple covalent bonds between atoms to satisfy the octet rule. (valence electrons are the electrons. Web steps of drawing o2 lewis structure step 1: However those all steps are. Oxygen belongs to the 16 th group of the periodic table and has 6 valence electrons. The test also checks the balance of acids and bases, known as the ph balance, in your blood. First of all, we calculate the total valence electrons for the o2. Web the lewis dot structure of beryllium and oxygen is relatively simple. Web drawing the lewis structure for o. Use these steps to correctly draw the o 2 lewis structure: Double covalent bonds are forming in an o2 molecule. Find the total valence electrons in o2 molecule. Let’s try to draw the o2 lewis structure in a few steps with an explanation. Some molecules must have multiple covalent bonds between atoms to satisfy the octet rule. Valence electrons are the most important things to draw lewis structure. Small nonpolar substances tend to be gasses. Web this widget gets the lewis structure of chemical compounds. Web 6 steps to draw the lewis structure of o2 step #1: Web drawing the lewis structure for o 2 ( dioxygen or oxygen gas) oxygen (o 2) is a commonly tested lewis structure due to it's importance on earth. Find how many electrons are needed: The test also checks the balance of acids and bases, known as the ph balance, in your blood. It is two for each oxygen atom. The o 2 lewis structure indicates that the o 2 molecule is perfectly symmetric. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable. When we draw a lewis structure, there are several guidelines to follow. A lewis structure shows the bonding and nonbonding electrons around individual atoms in a molecule. It also is a good example of a molecule with a double bond. (valence electrons are the electrons that are present in the outermost orbit of any atom.).
How to draw dot and cross diagram of Oxygen molecule YouTube

How to Draw the Lewis Dot Structure for O2 Oxygen gas YouTube
How to Draw O2 Lewis Structure? 3
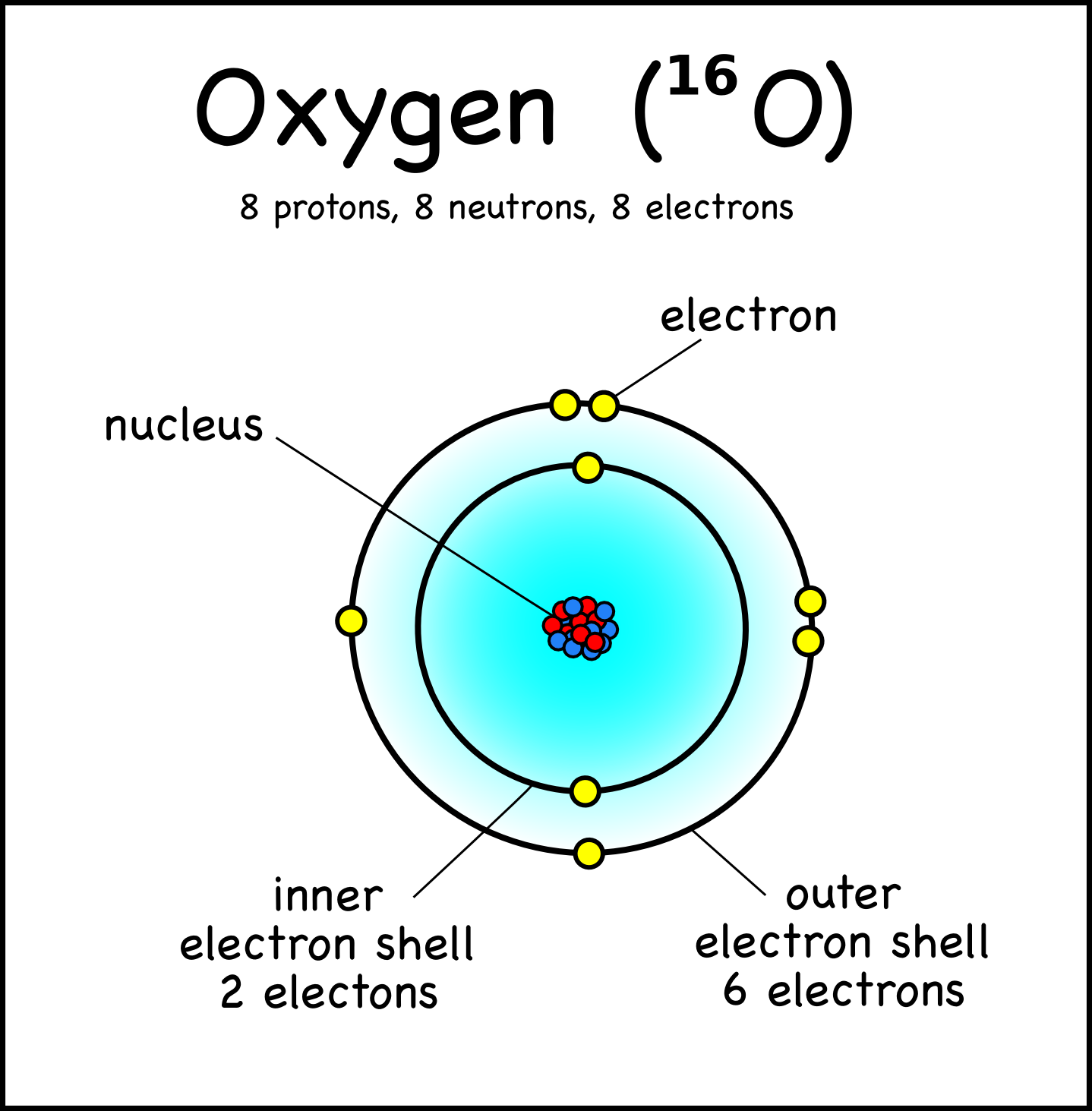
Bohr Model Drawing Of Oxygen at GetDrawings Free download

O2 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist

O2 oxygen molecule Royalty Free Vector Image VectorStock

Oxygen molecule, illustration Stock Image C038/8416 Science Photo

O2 oxygen molecule Royalty Free Vector Image VectorStock

Diagram representation of the element oxygen Vector Image

O2 2 Lewis Structure How to Draw the Lewis Structure for O2 2 YouTube
Web Steps Of Drawing Lewis Structure Of O 2 Molecule.
Look For The Total Number Of Bonds Forming:
Web Drawing The Lewis Structure For O.
In Order To Find The Total Valence Electrons In O2 (Oxygen) Molecule, First Of All You Should Know The Valence Electrons Present In A Single Oxygen Atom.
Related Post: