Metallic Bonding Drawing
Metallic Bonding Drawing - Lewis diagram of formaldehyde (ch₂o) worked example: The bonding worksheets cover the following topics: It creates a bulk of metal atoms, all clumped together. Delocaized electrons are free to move in the metallic lattice. An example of this is a copper wire or an aluminum sheet. Metallic bonding is bonding between metal ions in a metal. Lewis diagram of xenon difluoride (xef₂) exceptions to the octet rule. Vsepr for 2 electron clouds. This accounts for the high malleability and ductility of metals. In a metal, the stationary metal cations are surrounded by a sea of mobile valence electrons. Single and multiple covalent bonds. There are free electrons available to move through the structure and carry charge. But because a neutral sodium has one valence electron, we would just draw that one valence electron like that. Aluminum foil, copper wires), or it may be a mixture of two or more. In an alloy, the atoms are different sizes which. Metallic bonds occur among metal atoms. Using formal charges to evaluate nonequivalent resonance structures. Magnesium has the outer electronic structure 3s 2. Metallic bonds are the strong electrostatic attractions between the positively charged metal ions and the delocalised electrons. There are many strong metallic bonds in giant metallic structures between the positive metal ion and delocalised electrons. There are many strong metallic bonds in giant metallic structures between the positive metal ion and delocalised electrons. Atomic cores immersed in a valence electron fluid. Aluminum foil, copper wires), or it may be a mixture of two or more. Web because each ion is surrounded by the electron fluid in all directions, the bonding has no directional properties; Scaffolded,. Web a metallic bond is the attraction of the stationary metal cations to the surrounding mobile electrons. In an alloy, the atoms are different sizes which distorts the layered structure. This is sometimes described as an array of positive ions in a sea of electrons. This accounts for the high malleability and ductility of metals. What is this characteristic best. A metallic substance may be a pure element (e.g. Atomic cores immersed in a valence electron fluid. Web because each ion is surrounded by the electron fluid in all directions, the bonding has no directional properties; Web the more protons the stronger the bond 2. Single and multiple covalent bonds. Metallic bonding example magnesium has stronger metallic bonding than sodium and hence a. Delocalised electrons are free to move throughout the whole. Web because each ion is surrounded by the electron fluid in all directions, the bonding has no directional properties; When the metal atoms are in lattice structures, the electrons in their outer shells are free to move throughout. Predicting bond type (metals vs. Lewis diagram of formaldehyde (ch₂o). Metallic bonds occur among metal atoms. When sodium atoms arrange together, the outermost electron of one atom shares space with the corresponding electron on a neighboring atom. A metallic substance may be a pure element (e.g. Single and multiple covalent bonds. Predicting bond type (metals vs. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Is the attraction between the positive ions in a regular lattice and the. Now let's go to the other end of the periodic table. Delocalised electrons are free to move throughout the whole. Metals tend to form cations. When there are many of these cations, there are also lots of electrons. Scaffolded, partially scaffolded and unscaffolded. Web metals have high melting and boiling points. You will find model answers to all levels of these worksheets in the teacher guidance. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons. Web metallic bonding in magnesium. An example of this is a copper wire or an aluminum sheet. Web the metallic bond is commonly observed in metals. Identifying ionic, covalent and metallic bonds. The metal is held together by the strong forces of attraction between the positive nuclei and the delocalized electrons. A third major type of chemical bonding is metallic bonding. There are free electrons available to move through the structure and carry charge. Web metals have high melting and boiling points. Metallic bonds are the strong electrostatic attractions between the positively charged metal ions and the delocalised electrons. Using formal charges to evaluate nonequivalent resonance structures. When drawing a diagram of a metal’s structure, be sure to draw the ions in regular rows. Lewis diagram of the cyanide ion (cn⁻) worked example: This is sometimes described as an array of positive ions in a sea of electrons. A lot of heat energy is needed to break these bonds. Web the ability to conduct electricity in the solid state is a characteristic of metallic bonding. Web the bonding worksheets cover covalent, ionic and metallic bonding, available with three levels of support: Predicting bond type (metals vs. Formal charge and dot structures. Metal atoms are tightly packed together in lattice structures.
Metals
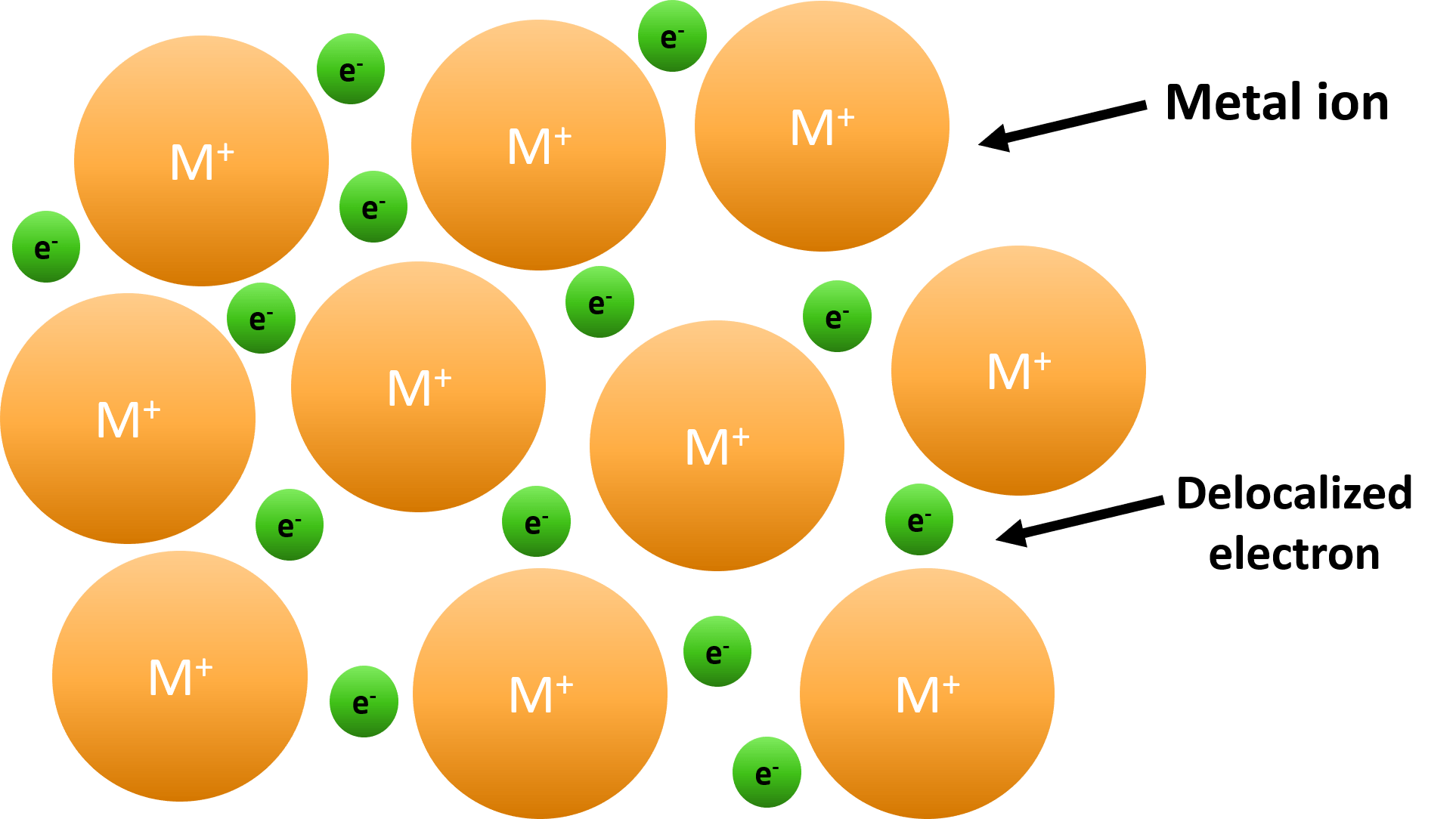
Metallic Bond — Formation & Compounds Expii

What is a metallic bond and how does it form Metallic Bonding
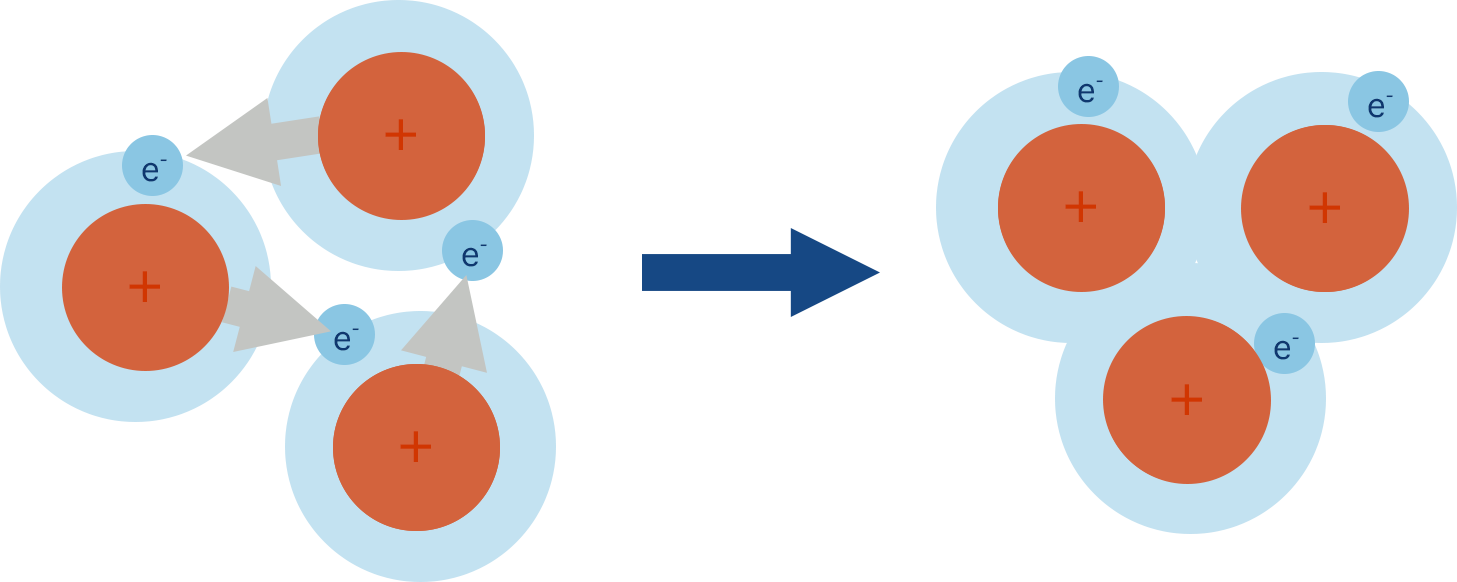
Metallic Bonding (ALevel) ChemistryStudent

Metallic bonding & giant metallic structure O Level Chemistry Notes
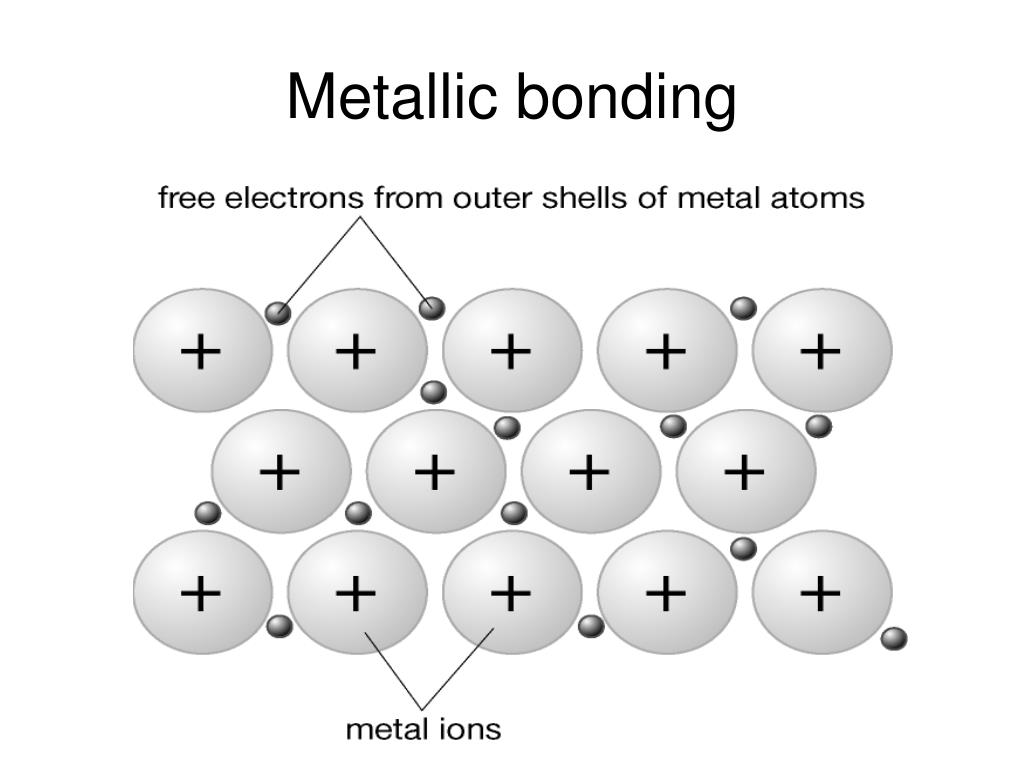
PPT Metallic bonding and properties PowerPoint Presentation, free
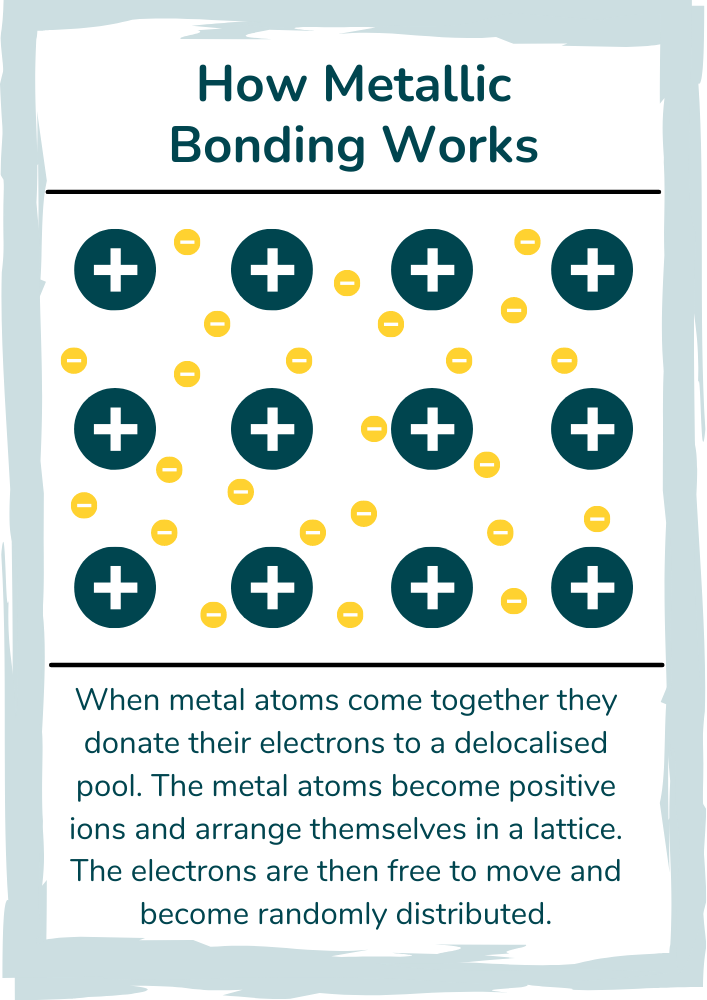
Metallic Bonding Explained Discover Tutoring
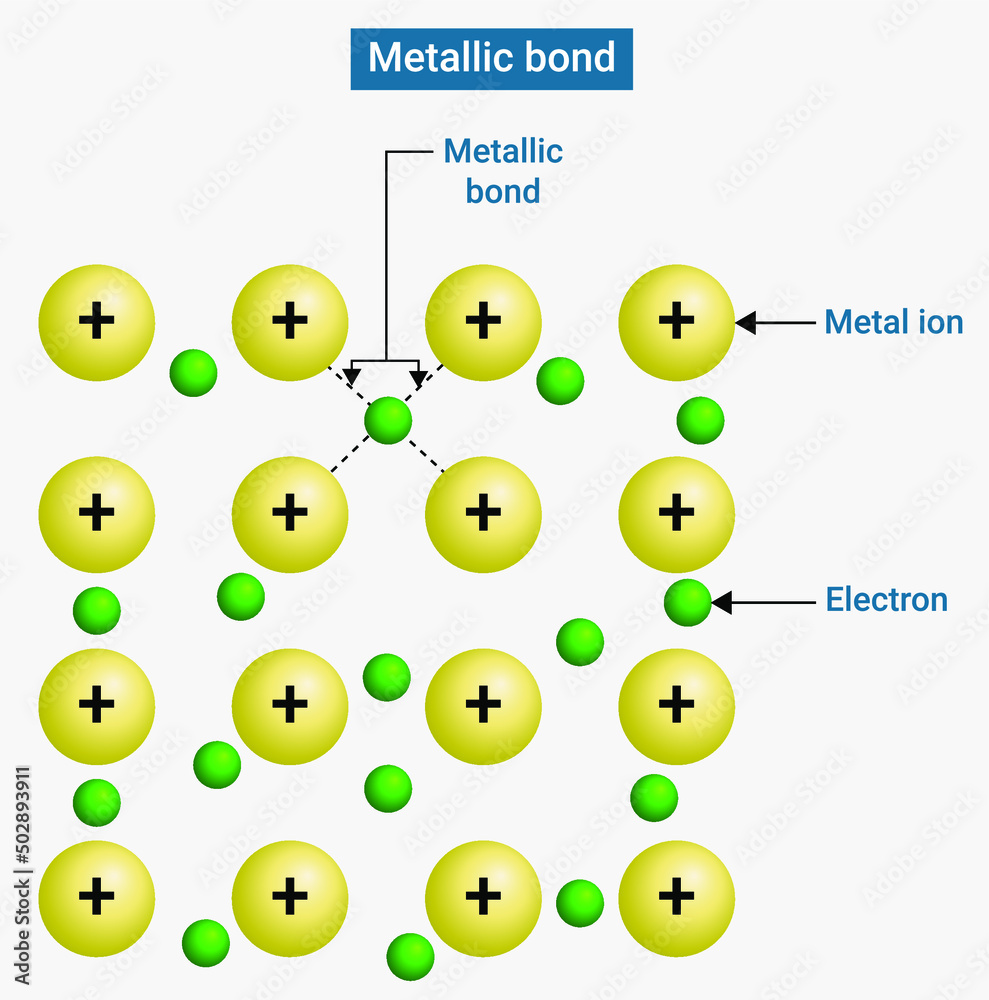
Metallic bond Metallic bonding is the electrostatic attractive force

Metallic Bonding GCSE Chemistry Science) AQA Revision

Bonding and Structure Edexcel T1 revisechemistry.uk
Single And Multiple Covalent Bonds.
The Remaining Ions Also Have Twice.
Atomic Cores Immersed In A Valence Electron Fluid.
A Metallic Substance May Be A Pure Element (E.g.
Related Post: