Master Validation Plan Template
Master Validation Plan Template - 2.5 tools, techniques, and methodology. Web validation master plan template. 3 verification and validation plans. Web master validation plan template for medical devices: Web fda quality systems regulations. The purpose of this guideline is to provide guidance on the preparation of validation master plans (vmp). Introduction to master validation plans. Web preview validation master plan template. In this comprehensive guide, we’ll address key questions such as. The document is fully editable so that you can adapt it to your company design. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). Web the validation master plan (vmp) is a summary of the planned validation activities. The document is fully editable so that you can adapt it to your company design. Whether you're setting out to develop a vmp or seek to identify. It lists those activities and essential documents which will be generated and defines staff responsibilities. Web the validation master plan (vmp) is a summary of the planned validation activities. Web the validation master plan is a summary of validation strategy. In this comprehensive guide, we’ll address key questions such as. 3 verification and validation plans. In this comprehensive guide, we’ll address key questions such as. Home › complianceonline standards › fda validation › validation master plan template. Web a quick validation master plan checklist. Web 2.2 scope of the document. The document is fully editable so that you can adapt it to your company design. Web a quick validation master plan checklist. Documents include placeholder marks for all. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. 3 verification and validation plans. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a. Web fda software validation template. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Web the validation master plan is a summary of validation strategy. Web fda quality systems regulations. The purpose of the validation master plan is to document the compliance requirements for the site. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Web preview validation master plan template. Master validation plans are an essential part. The purpose of the validation master plan is to document the compliance requirements for the site and to. As it is a summary, it. Web fda quality systems regulations. Web 2.2 scope of the document. Master validation plans are an essential part. Documents include placeholder marks for all. Introduction to master validation plans. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. The purpose of the validation master plan is to document the compliance requirements for the site and to. Web 2.2 scope of the document. Master validation plans are an. Web 2.2 scope of the document. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. The document is fully editable so that you can adapt it to your company design. 2.5 tools, techniques, and methodology. Web the validation. As it is a summary, it does not repeat information documented in validation protocols or standard operating procedures. Whether you're setting out to develop a vmp or seek to identify weaknesses in an existing one, the following questions. Web 2.2 scope of the document. Master validation plans are an essential part. 2.5 tools, techniques, and methodology. The purpose of the validation master plan is to document the compliance requirements for the site and to. Web fda software validation template. 3 verification and validation plans. A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation. Introduction to master validation plans. In this comprehensive guide, we’ll address key questions such as. Master validation plans are an essential part. Web a validation master plan, also referred to as “vmp”, outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides a. Web a free master validation plan (mvp) form to help medical device manufacturers with documenting a list of all company processes requiring validation. Web preview validation master plan template. Documents include placeholder marks for all. It lists those activities and essential documents which will be generated and defines staff responsibilities. Web the validation master plan (vmp) is a summary of the planned validation activities. Web at the core of the validation process is a fundamental document known as a validation master plan (vmp). As it is a summary, it does not repeat information documented in validation protocols or standard operating procedures. The document is fully editable so that you can adapt it to your company design.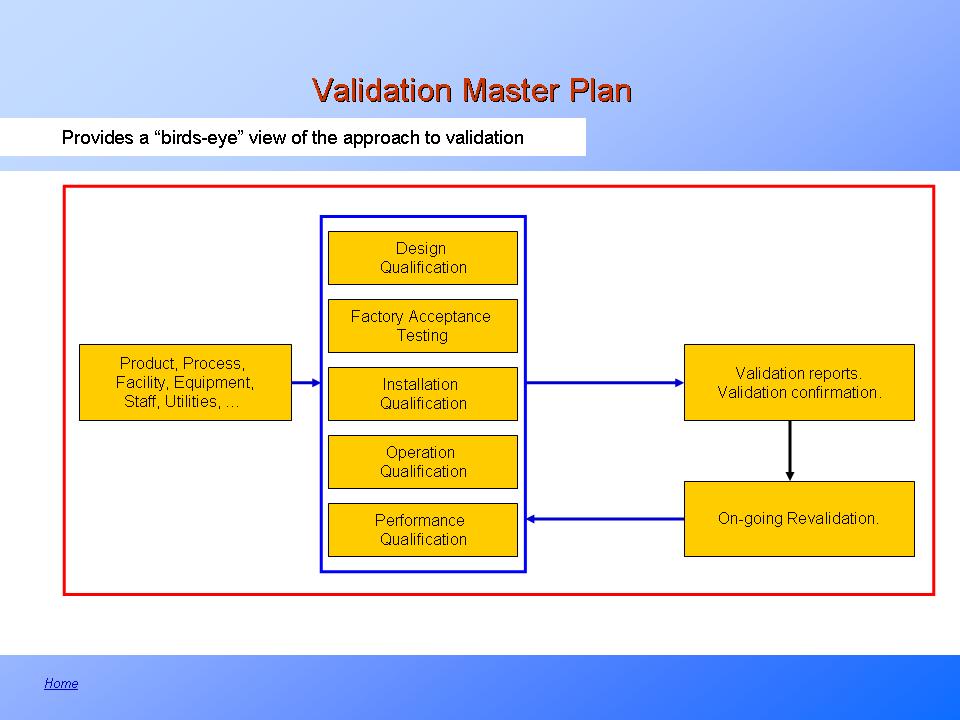
Overview of the Validation Master Plan PresentationEZE
Validation Master Plan Bio Chem Shop

Nine steps for creating a Master Validation Plan

FREE 9+ Sample Validation Plan Templates in PDF MS Word
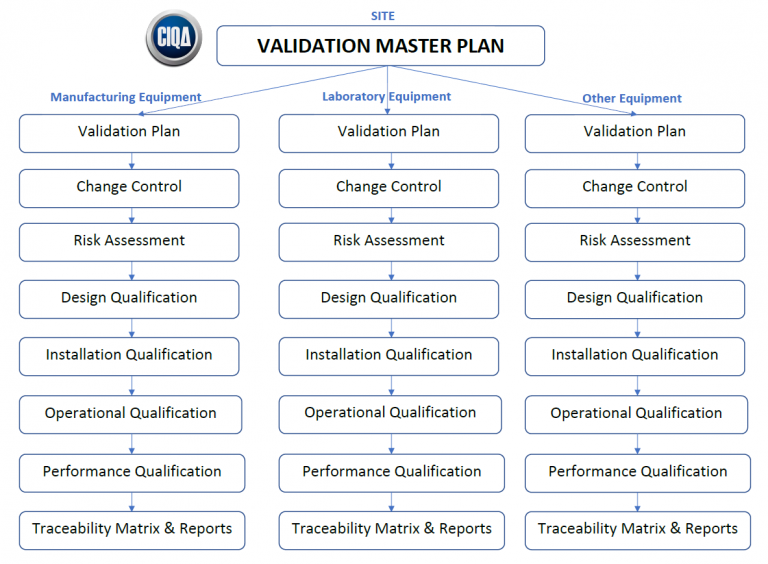
How to create a Validation Master Plan in 5 steps. Templates & more

10+ Validation Plan Templates Sample Templates
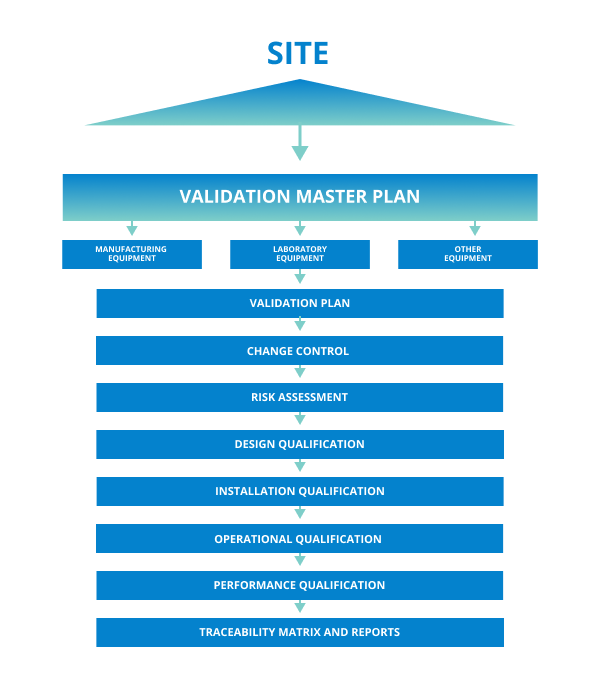
Validation Master Plan Egnyte

Validation Master Plan Template Validation Center
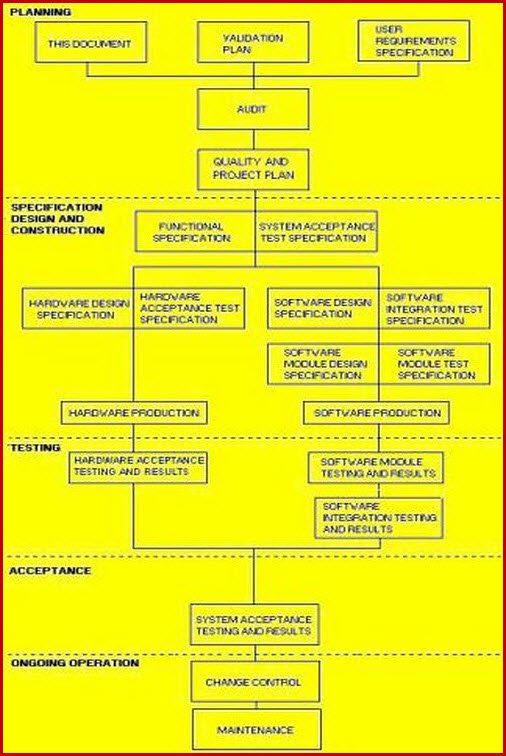
Validation Master Plan (VMP) Downloadable Interactive Template.
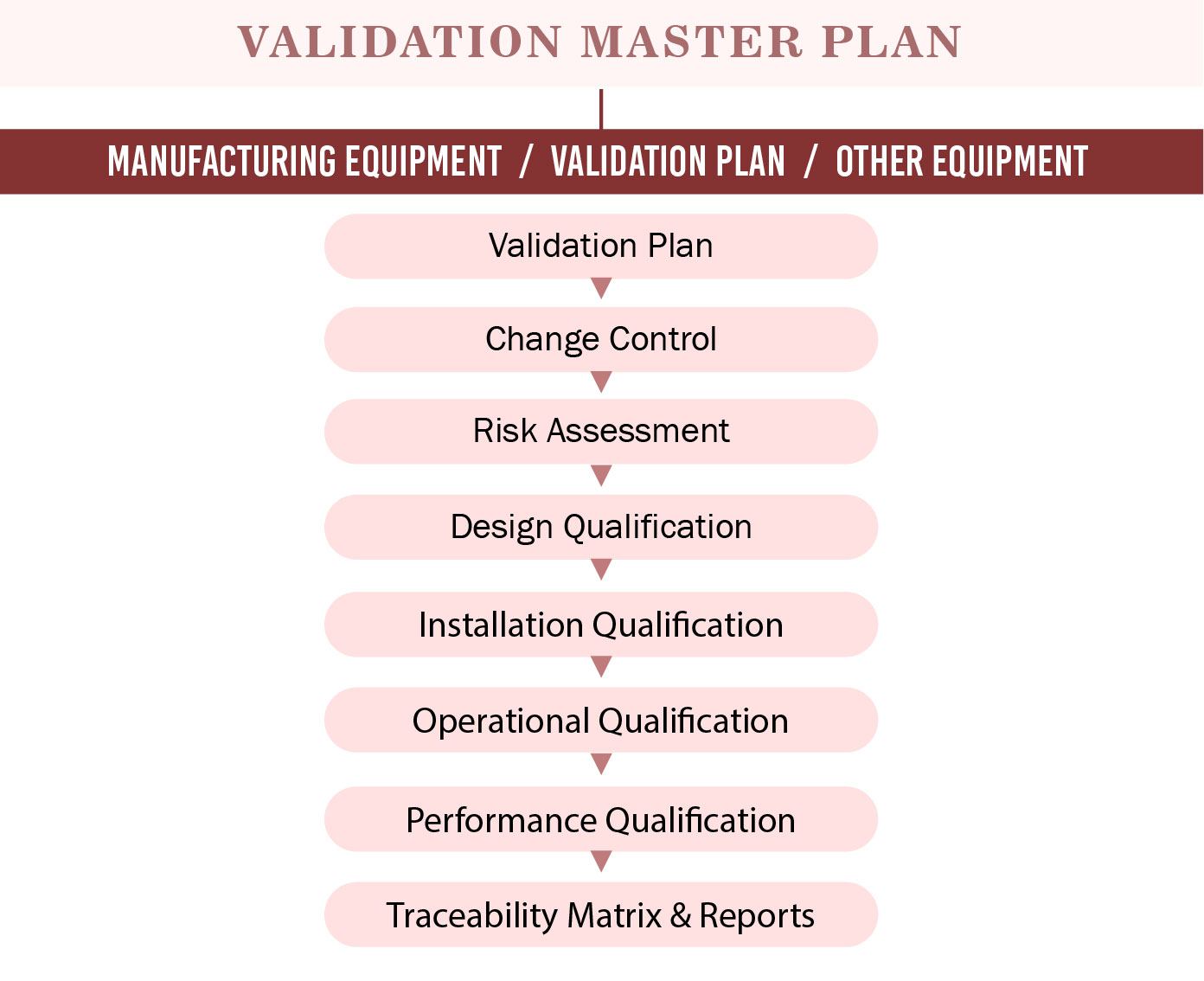
Validation Master Plan Template
Web 2.2 Scope Of The Document.
Web Validation Master Plan Template.
Software Validation For The Chemical, Manufacturing And Cannabis Industries.
2.5 Tools, Techniques, And Methodology.
Related Post: