Master Formulation Record Template
Master Formulation Record Template - Web master formulation and compounding records (1) creating master formulation records » any changes or alterations to the master formulation records. A batch manufacturing record (bmr) is an important document for chemical and process manufacturers: Medium risk level (batch compound) in a refrigerator: Documentation—ingredients with ndc or api codes: The following is a best practice recommendation on the elements of a. Web for the complete list, please refer to the guidance document as well as the acp mfr template that may be used to complete your mfrs. Web step by step procedure to write a master formula record (mfr) for pharmaceutical products. Web master formulation record is recommended when performing batch or high risk compounding. Web a master formulation record is maintained for each batch of prepared csps and includes the following information: It tells users how to produce a batch. Web a master formulation record is maintained for each batch of prepared csps and includes the following information: Web for the complete list, please refer to the guidance document as well as the acp mfr template that may be used to complete your mfrs. Master formulation records are required for drugs compounded in batches or any compound that is made. Web for the complete list, please refer to the guidance document as well as the acp mfr template that may be used to complete your mfrs. Web printable and fillable forms. Master formula record (mfr) is a master document for any. Web march 4, 2024 by caitlin o'donnell. Web sample master formulation record. A batch manufacturing record (bmr) is an important document for chemical and process manufacturers: Web for the complete list, please refer to the guidance document as well as the acp mfr template that may be used to complete your mfrs. Web master formulation record page 1 of 2 december 7, 2020 template template master formulation record. Master formula record (mfr). Web master formulation record vs compounding record; Preparation name, strength, and dosage form. Web sample master formulation record. Master formulation records are required for drugs compounded in batches or any compound that is made more than once. Documentation—ingredients with ndc or api codes: Web a master formulation record should be created compounding—sterile preparations 〈797〉, pharmaceutical before compounding a preparation for the first time. Web printable and fillable template for a master formulation record (napra) pharmacy connection ~ winter/spring 2021 ~ page 21. Master formula record (mfr) is a master document for any. Web master formulation record vs compounding record; Web sample master formulation. Web step by step procedure to write a master formula record (mfr) for pharmaceutical products. Master formulation records are required for drugs compounded in batches or any compound that is made more than once. Web master formulation record vs compounding record; Web march 4, 2024 by caitlin o'donnell. Medium risk level (batch compound) in a refrigerator: Master formula record (mfr) is a master document for any. Web printable and fillable template for a master formulation record (napra) pharmacy connection ~ winter/spring 2021 ~ page 21. Web template 2 master formulation record. Medium risk level (batch compound) at controlled room temperature: Web a master formulation record is maintained for each batch of prepared csps and includes the. Web for the complete list, please refer to the guidance document as well as the acp mfr template that may be used to complete your mfrs. Web template 2 master formulation record. Hydrocortisone 1% / ketoconazole 2% 1:1. The following is a best practice recommendation on the elements of a. Medium risk level (batch compound) in a refrigerator: Hydrocortisone 1% / ketoconazole 2% 1:1. Web printable and fillable forms. Preparation name, strength, and dosage form. Documentation—ingredients with ndc or api codes: Web master formulation record is recommended when performing batch or high risk compounding. It tells users how to produce a batch. Web a master formulation record should be created compounding—sterile preparations 〈797〉, pharmaceutical before compounding a preparation for the first time. Web master formulation and compounding records (1) creating master formulation records » any changes or alterations to the master formulation records. Documentation—ingredients with ndc or api codes: Web printable and fillable forms. Web pharmaceutical form route of administration developed by verfied by formula ingredients quantities physical description other information (i.e din, unique identifier such as cas,. Hydrocortisone 1% / ketoconazole 2% 1:1. Documentation—ingredients with ndc or api codes: Web master formulation record is recommended when performing batch or high risk compounding. Web sample master formulation record. Web for the complete list, please refer to the guidance document as well as the acp mfr template that may be used to complete your mfrs. Download or create your own template for the master formulation record. Web printable and fillable template for a master formulation record (napra) pharmacy connection ~ winter/spring 2021 ~ page 21. Master formulation records are required for drugs compounded in batches or any compound that is made more than once. Web master formulation record page 1 of 2 december 7, 2020 template template master formulation record. Medium risk level (batch compound) at controlled room temperature: Web master formulation record vs compounding record; Web printable and fillable forms. Web march 4, 2024 by caitlin o'donnell. Master formula record (mfr) is a master document for any. Preparation name, strength, and dosage form.
PPT Batch Manufacturing Record and Master Formula Record PowerPoint
Master Formula Record Sample PDF PDF

Master Formula Record (MFR) Solution Parmacy

MFR (master formula record )
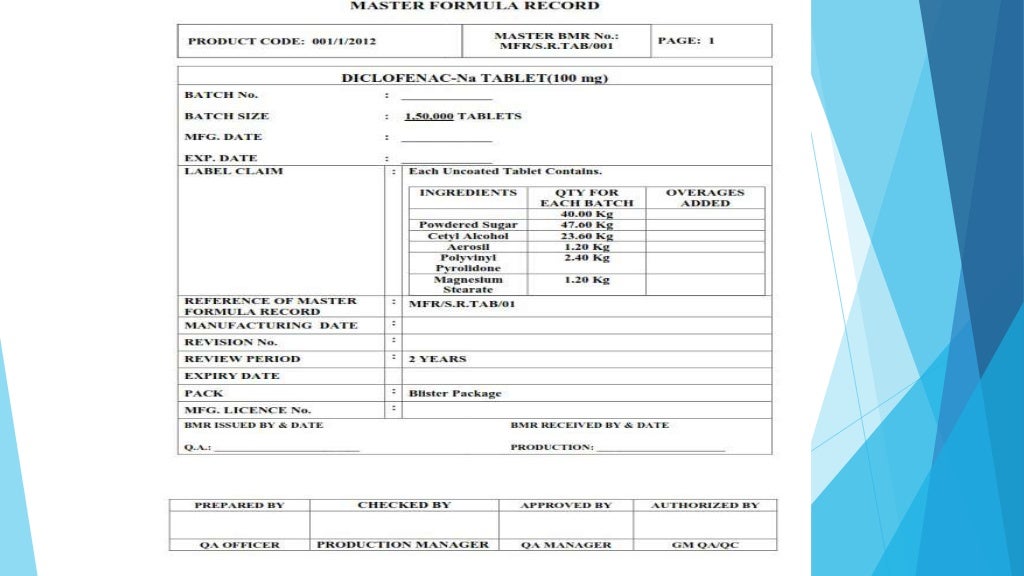
Master formula record

Usp 797 Master Formulation Record Template

Usp 797 Master Formulation Record Template
Tempate Master Formulation Record Compounding PDF

Master Formula Record (MFR) Pharmaceutical Quality Assurance Unit4
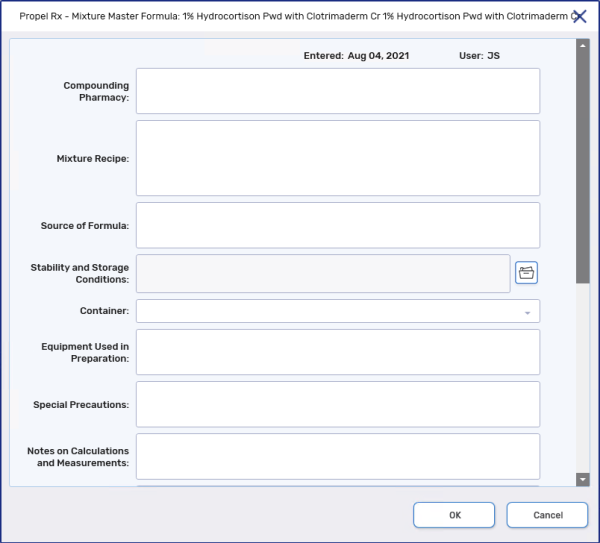
Master Formulation Record Overview
Web Template 2 Master Formulation Record.
Web Master Formulation And Compounding Records (1) Creating Master Formulation Records » Any Changes Or Alterations To The Master Formulation Records.
Medium Risk Level (Batch Compound) In A Refrigerator:
It Tells Users How To Produce A Batch.
Related Post:

