Irb Template
Irb Template - It is recommended to use templates that incorporate. Web template name version number version date; Exempt consent templates and guidance. Find the new informed consent guidance resources here. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for. Highlighting sample irb templates and submission documents. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. Web new for fall 2023: Sample templates and submission documents. Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): It is recommended to use templates that incorporate. Sop irb member standards and responsibilities. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. National cancer institute (nci) / central institutional review board (cirb) usc required informed consent. To determine if you need to consent your subjects to be screened. Web the irb review process and time vary depending on the level of review required. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for. Web information sheet for exempt studies. Find the new informed consent guidance resources here. Find protocol templates for human research activities at northwestern university, such. To aid in the development of research. Web template name version number version date; Assent templates and assent information. For use when submitting new applications to be initially approved after. National cancer institute (nci) / central institutional review board (cirb) usc required informed consent. Sop irb member standards and responsibilities. Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. Our forms and guidance documents are often updated. Sample templates and submission documents. Find the new informed consent guidance resources here. To register an irb if your institution or organization has not previously registered an irb. Sop irb member standards and responsibilities. For use when submitting new applications to be initially approved after. Below are templates of commonly needed submission documents. Web the templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. Assent templates and assent information. Always download fresh forms and templates with each new irb submission! Our forms and guidance documents are often updated. Some ancillary reviews must be completed prior. Sample templates and submission documents. Web investigators should use the protocol and consent templates that are available on the ohsrp website irb templates page. To update or renew the registration of. Assent templates and assent information. Web new for fall 2023: To determine if you need to consent your subjects to be screened (in person, on the phone, or online), please see the section at. Find protocol templates for human research activities at northwestern university, such as biomedical, social and behavioral, and data and specimen analysis. Web template name version number version date; Sample templates and submission documents. Our forms and. Exempt consent templates and guidance. As of may 17th, 2023, the icf templates available on our informed consent templates page replaces all previously released templates. Web human subjects (irb) irb protocol submission; National cancer institute (nci) / central institutional review board (cirb) usc required informed consent. Below are templates of commonly needed submission documents. Sample templates and submission documents. Our forms and guidance documents are often updated. To aid in the development of research. Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): Web screening consent template. The following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for. Web the irb review process and time vary depending on the level of review required. Below are templates of commonly needed submission documents. To aid in the development of research. National cancer institute (nci) / central institutional review board (cirb) usc required informed consent. Web screening consent template. Find the new informed consent guidance resources here. To register an irb if your institution or organization has not previously registered an irb. Exempt consent templates and guidance. Always download fresh forms and templates with each new irb submission! To determine if you need to consent your subjects to be screened (in person, on the phone, or online), please see the section at. Web jhm irb combined informed consent/hipaa authorization template (march 2023, version 17): Find protocol templates for human research activities at northwestern university, such as biomedical, social and behavioral, and data and specimen analysis. Sample templates and submission documents. It is recommended to use templates that incorporate. For use when submitting new applications to be initially approved after.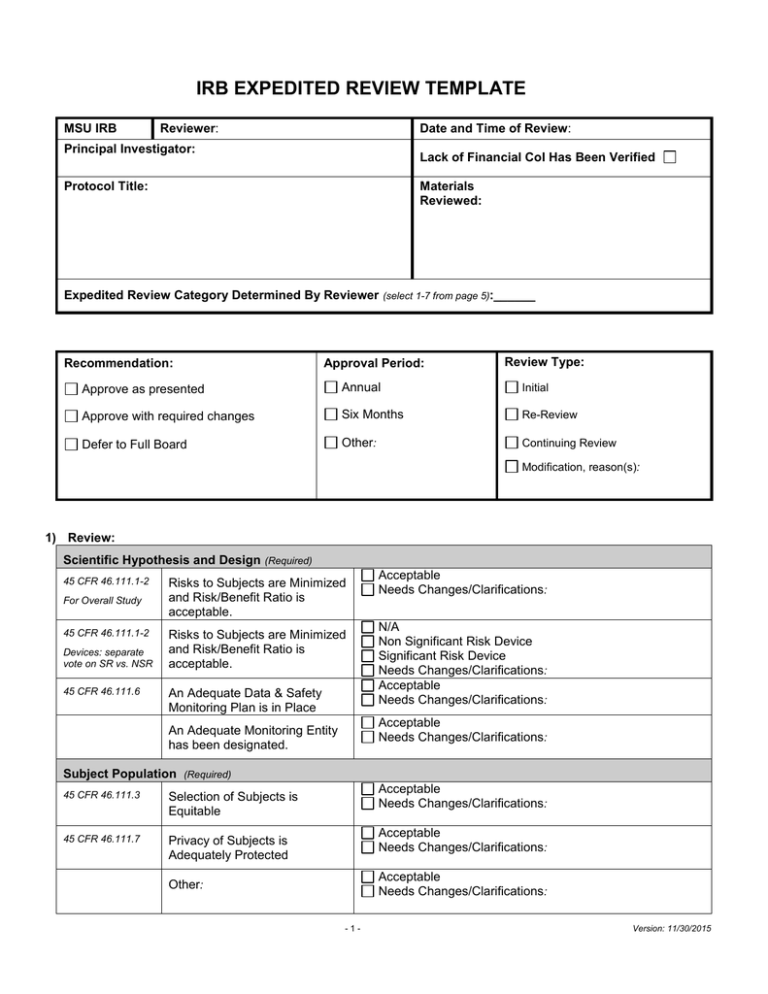
IRB EXPEDITED REVIEW TEMPLATE

28 Irb Form Templates free to download in PDF

Form IRB2 Fill Out, Sign Online and Download Printable PDF, New

IRB Proposal Template FIU Research

Irb Template Form Fill Out and Sign Printable PDF Template airSlate

Top 7 Irb Consent Form Templates free to download in PDF format

IRB authorization agreement in Word and Pdf formats

Research Pdf 10421 Cu Irb Flyer Template Flyer Template Word

Request For Non Human Subject Research or IRB Exemption Doc Template

Form IRB1 Fill Out, Sign Online and Download Printable PDF, New
Highlighting Sample Irb Templates And Submission Documents.
Learn How To Use The Consent Form Wizard For Educational And Social.
To Update Or Renew The Registration Of.
Our Forms And Guidance Documents Are Often Updated.
Related Post: