Irb Protocol Template
Irb Protocol Template - Submission to the hrpp for review of institutional requirements and irb for review for the approval criteria may require different documents. Web post the version of the protocol that was originally approved and is relevant to the study at hand. Irb application form 6.4 irb revision request form The reliance agreement templates below, such as the iaa, are specific to ohsu waiving oversight. This tool asks a series of questions to. The irb toolkit’s registry and repository protocol template is the most efficient way for you to provide the information the irb needs. In the get started section, complete the following: Under certain circumstances, it is recommended that researchers use a research protocol template in preparation for submitting their study via the new protocol submission xform in irbmanager.refer to the document below for. The protocol must also comply with current fda electronic submission requirements. Web which protocol template and protocol checklist should i use? Web the irb provides several protocol templates on this page. Additional irb templates are provided to promote transparency of irb operations. Web •for ceded studies using a central irb (wcg/advarra/sterling irb), there will be a protocol available from the sponsor that you will use. A template used when submitting. Choose a different pi, if necessary. Web the following template provides essential topics of interest that the irb looks for during review. Common examples of research resources are a data or biospecimen repository and a recruitment database. Additional irb templates are provided to promote transparency of irb operations. Web the irb provides several protocol templates on this page. Authorization to submit to neals irb (doc) 8/3/20: Web the irb office has developed the following research protocol template for use by the nsu research community. If your department does not have a standard protocol template, you. There are two templates (and accompanying instructions for each) to be used for exempt research, one for prospective data collection. Listed below are several templates to assist you in composing your. Document headerthe document header contains the h s d u w logo, the document type, and the document title. The generic protocol template available on the protocol templates and guidelines page provides an example of how this should look. Additional irb templates are provided to promote transparency of irb operations. In the get started section, complete the following: They follow. Use this irb review application if you have completed the therapeutic studies jcto protocol template and/or have a study which will use a device/drug or implement a clinical trial. The natural history/observational protocol template, the repository protocol template, and the secondary research protocol template. Web the following protocol and consent templates are used by researchers in preparation for irb submission. Web irb application for human participant research use this form to submit a new human subject research protocol to the irb. Remove all template guidance before you attach the file to the submission. Web for a protocol not authored by cpws, ctep strongly urges the pi to use the protocol template provided with the full loi approval letter. Hipaa and. Web there are three templates to be used for observational research: Web the following protocol and consent templates are used by researchers in preparation for irb submission (see investigator manual (download) for additional guidance on completing these documents). This form replaces the old adobe pdf form. Visit tc irb’s website/how to submit/guides/existing data for more information. Web post the version. They follow the format of typical nih and industry multicenter protocols. A template used when submitting to neals irb. This irb review application template is only to be used for the establishment of a biorepository (storage and maintenance) for. If your department does not have a standard protocol template, you. Instructions specific to items on the templates appear in red. Submission to the hrpp for review of institutional requirements and irb for review for the approval criteria may require different documents. Hipaa and data use agreements are not part of the toolkit and can be found. Web the irb provides several protocol templates on this page. Studies that will be reviewed by an. Web this section contains templates, forms, and. Web •for ceded studies using a central irb (wcg/advarra/sterling irb), there will be a protocol available from the sponsor that you will use. Web for a protocol not authored by cpws, ctep strongly urges the pi to use the protocol template provided with the full loi approval letter. While these studies are likely to be minimal risk, they must include. Web the irb office has developed the following research protocol template for use by the nsu research community. Human subjects protection program/irb 4 Web which protocol template and protocol checklist should i use? Document headerthe document header contains the h s d u w logo, the document type, and the document title. Attached below is a model template. Web promotion rules template with instructions all promotions should include official rules addressing eligibility, deadlines, odds, sponsor information, and amoe. Instructions specific to items on the templates appear in red text in brackets. This part of the protocol deals specifically with existing data. This tool asks a series of questions to. The natural history/observational protocol template, the repository protocol template, and the secondary research protocol template. Web be met through other means. Irb application form 6.4 irb revision request form Web from the irb menu, select create irb protocol to start a new protocol. Wcg authorization form (docx) 11/15/23: Web listed below are several templates to assist investigators in creating and submitting their research protocols to the yale irb. Web visit tc irb’s website/how to submit/guides/writing for the irb for further tips.
Informed Consent Template Irb Master of Documents

Protocol Amendment IRB Submission . Doc Template pdfFiller

IRB PROTOCOL REVIEW EXAMPLE GUIDE

How to Submit an IRB Protocol

IRB Protocol Template Center for the Enhancement of Teaching

IRB authorization agreement in Word and Pdf formats

Irb Protocol Template
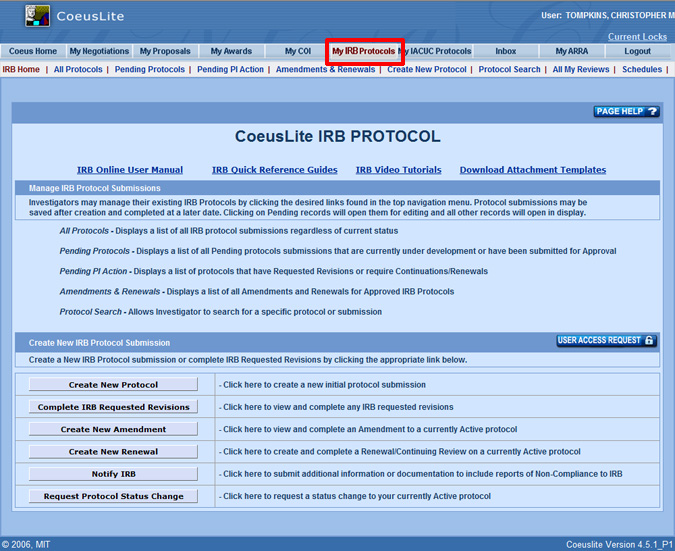
IRB Protocol Homepage Sponsored Program Services Coeus Purdue

Irb Protocol Template

example of an IRB Protocol
Every Submission Must Have A New Version Date.
Web This Section Contains Templates, Forms, And Guidance For Studies In Which The Ohsu Irb Is Waiving Oversight To An External Irb.
Assent Templates And Assent Information.
This Is A Microsoft Word Form With Fillable Fields.
Related Post: