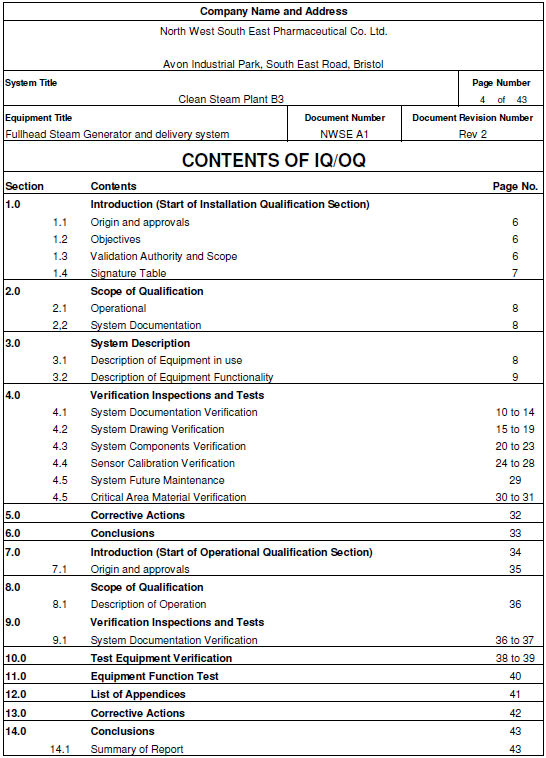
Iq Oq Pq Template
Iq Oq Pq Template - What is iq, oq, pq? Working for pharma company vs. Web write the objective of the protocol defining the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the equipment with location i.e., packaging or manufacturing, and the facility. Mounting qualification (iq), operational qualification (oq), and performance qualification (pq). These exist the abbreviations we use in one medical device industry used the three steps the process validation: Include a brief description of why these qualifications (iq/oq) are required i.e., new equipment. Installation qualification (iq), operational qualification (oq), and benefit proviso (pq). What the iq, oq, pq? Web what is iq, oq, pq? Equipment capability (iq) challenge conditions (oq) nominal operating What the iq, oq, pq? Web what is iq, oq, pq? Installation qualification (iq), operational qualification (oq), and performance qualification (pq). Web operational qualification (oq) performance qualification (pq) overcoming one of the biggest challenges to achieving iq, oq, pq success. These exist the abbreviations we use in one medical device industry used the three steps the process validation: Web the objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant number] which will be located in the [insert area, packaging or manufacturing] at site [insert site name]. What is iq, oq, pq? Use them right now to help with your qualification and validation projects. Installation qualification (iq), operational qualification (oq), and performance qualification (pq). What is iq, oq, pq? Installation qualification (iq), operational qualification (oq), and benefit proviso (pq). Web what is iq, oq, pq? Equipment capability (iq) challenge conditions (oq) nominal operating Include a brief description of why these qualifications (iq/oq) are required i.e., new equipment. Computer system validation (csv) gxp software systems; What the iq, oq, pq? Measuring iq, oq, pq success as a function of quality by design. These are the abbreviations we use in the medical device industry for the three steps of process validation: Installation qualification (iq) for hardware verifies that the physical equipment and ancillary systems are installed correctly and in accordance with manufacturer specifications and regulatory requirements. Web by the end of iq, oq and pq the following should be answered. Web what are iq oq pq?IQ, OQ, PQ Steam Quality Qualification Documentation
These Exist The Abbreviations We Use In One Medical Device Industry Used The Three Steps The Process Validation:
Working For Pharma Company Vs.
5 Validation Mistakes To Avoid;
Web Write The Objective Of The Protocol Defining The Installation Qualification (Iq) And Operational Qualification (Oq) Requirements And Acceptance Criteria For The Equipment With Location I.e., Packaging Or Manufacturing, And The Facility.
Related Post: