How To Draw Molecular Geometry
How To Draw Molecular Geometry - Methane isn't flat with 90° bond angles. Web using the cross bow arrow shown below we can show that it has a net dipole. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. Here is the list of protentional participants and their early odds to win. Pf 5 (phosphorus pentafluoride, a catalyst used in certain organic reactions) h 3 0 + (hydronium ion) given: A draw the lewis electron structure of the molecule or polyatomic ion. Determine the electron group arrangement around the central atom that minimizes repulsions. Write the lewis dot structure of the molecule. Web geometry of molecules. Web the 2024 preakness stakes field is beginning to take shape. Web how to use the table to predict molecular geometry. Methane isn't flat with 90° bond angles. Web this chemistry video tutorial provides a basic introduction into molecular geometry and vsepr theory. Molecules like ammonia have tetrahedral electronic geometry but trigonal pyramidal molecular geometry. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. From this we can describe the molecular geometry. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. Web the practice problems are intended for you to test yourself and see if you understand how to draw lewis dot structures and predict molecular geometries. Find out by adding single, double or triple bonds and lone pairs. How does molecule shape change with different numbers of bonds and electron pairs? Halogens and noble gases can expand their octet. Use the sn and vsepr theory to determine the electron pair geometry of the molecule. Consulting firm henley & partners identified 3 us cities with huge potential for wealth growth. Find out by adding single, double or triple bonds. Examples and practice problems include the trigonomal. Methane isn't flat with 90° bond angles. Dipole moment is equal to the product of the partial charge and the distance. The second figure serves as a visual aid for the table. To chose the central atom as the one with the smallest number of valence electrons or if they all have the. It applies a theory called vespr for short. Web how to use the table to predict molecular geometry. The vsepr model can be used to predict the shapes of many. Web the practice problems are intended for you to test yourself and see if you understand how to draw lewis dot structures and predict molecular geometries. Draw lewis structures for. Use the sn and vsepr theory to determine the electron pair geometry of the molecule. Web notice that the way the methane is drawn bears no resemblance to the actual shape of the molecule. Web the 2024 preakness stakes field is beginning to take shape. Then, compare the model to real molecules! To chose the central atom as the one. Web we recommend using the latest version of chrome, firefox, safari, or edge. Web using the cross bow arrow shown below we can show that it has a net dipole. Vespr stands for valence shell electron pair repulsion. Web this chemistry video tutorial provides a basic introduction into molecular geometry and vsepr theory. Web molecular geometries (linear, trigonal, tetrahedral, trigonal. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. It gives information about the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Μ = δ × d (3.7.1) (3.7.1) μ = δ × d. Web. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Web the 2024 preakness stakes field is beginning to take shape. It applies a theory called vespr for short. Molecules like ammonia have tetrahedral electronic geometry but trigonal. How does molecule shape change with different numbers of bonds and electron pairs? The table of molecular geometries can be found in the first figure. Web ketzbook explains molecular geometry, vsepr theory, and the 5 basic shapes of molecules with examples for each one.for a limited time, earn double free stock. A draw the lewis electron structure of the molecule. Explore molecule shapes by building molecules in 3d! Vespr stands for valence shell electron pair repulsion. Data that may be obtained from a molecule's geometry includes the relative position of each atom, bond lengths, bond angles, and torsional angles. It gives information about the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. The table of molecular geometries can be found in the first figure. Use the sn and vsepr theory to determine the electron pair geometry of the molecule. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. The table of molecular geometries can be found in the first figure. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Web geometry of molecules. That gives you the steric number (sn) — the number of bond pairs and lone pairs around the central atom. Web the 2024 preakness stakes field is beginning to take shape. Web this chemistry video tutorial provides a basic introduction into molecular geometry and vsepr theory. Consulting firm henley & partners identified 3 us cities with huge potential for wealth growth. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory.
C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
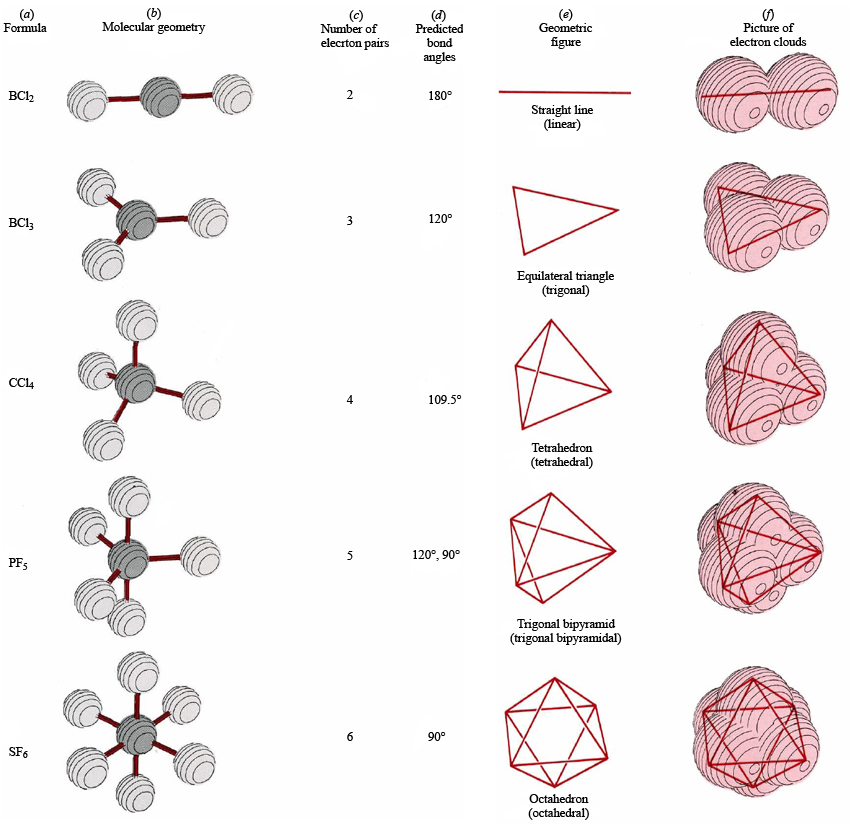
Molecular Geometry Chemistry Socratic
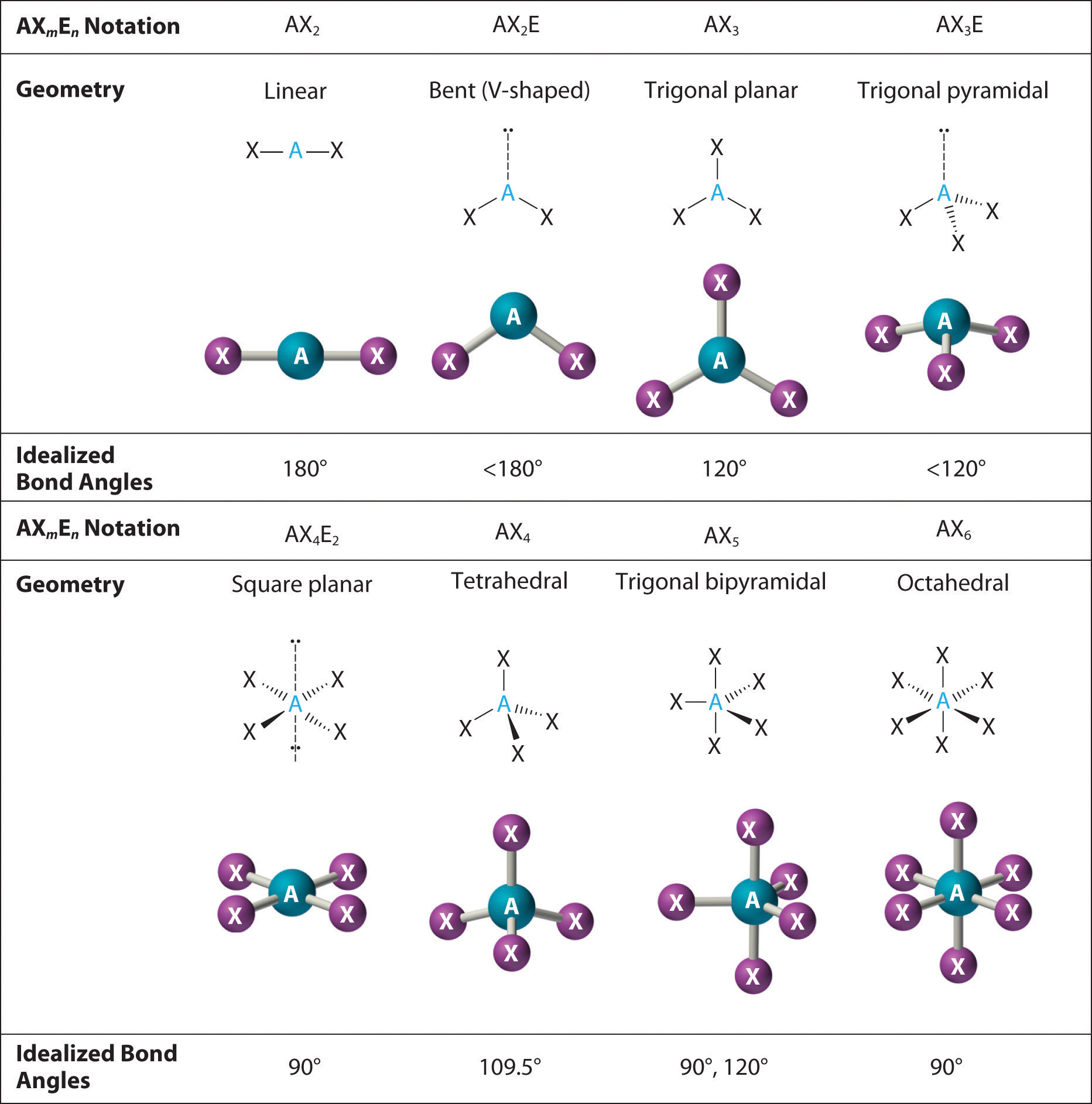
9.7 The Shapes of Molecules Chemistry LibreTexts

C2H2 Molecular Geometry / Shape and Bond Angles (see description for
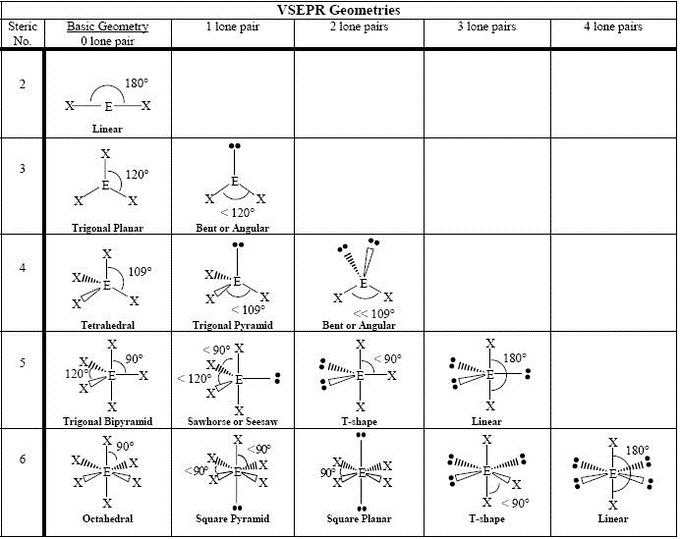
Molecular Geometry Introductory Chemistry

Molecular Geometry Boundless Chemistry
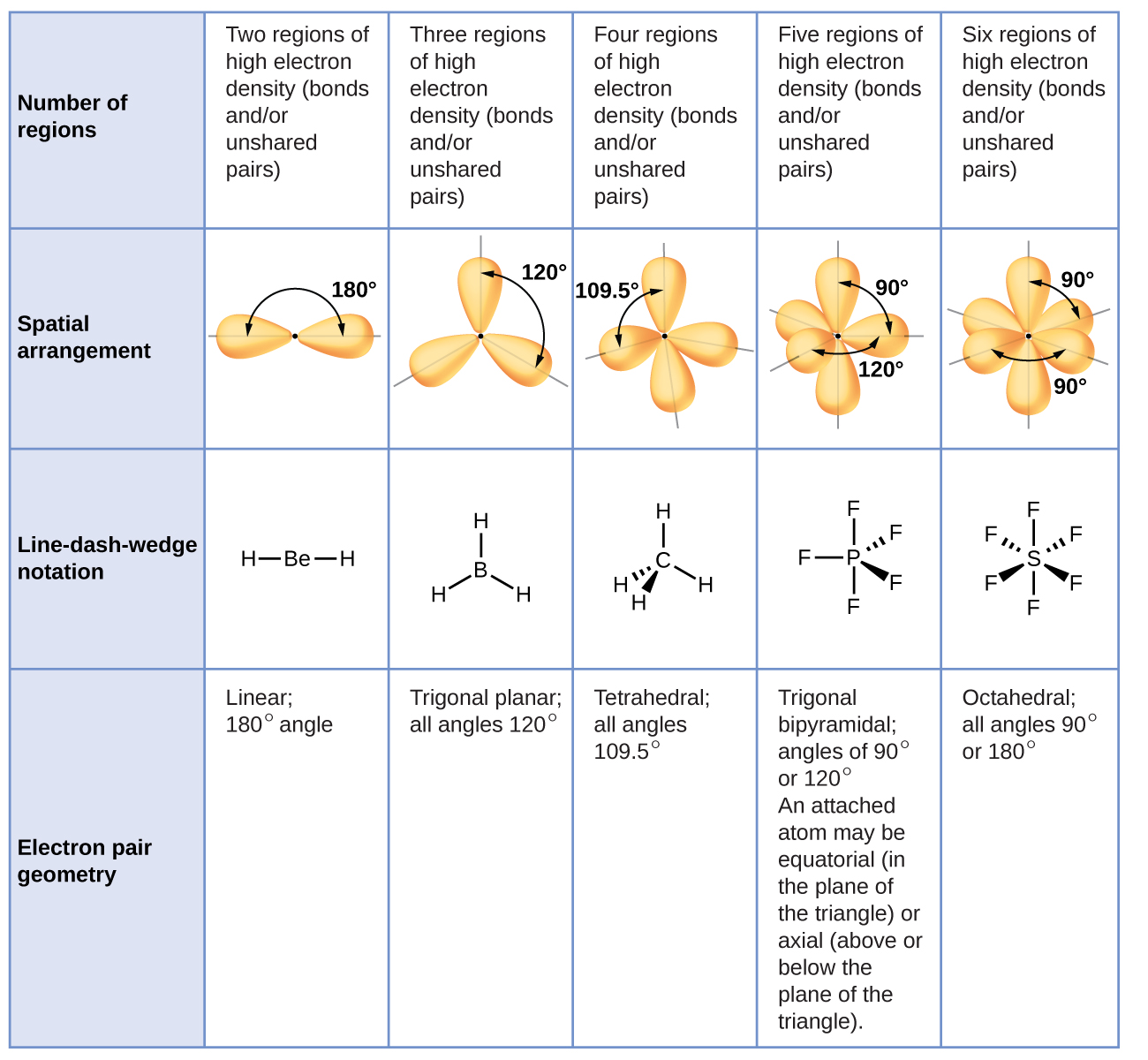
7.6 Molecular Structure and Polarity Chemistry
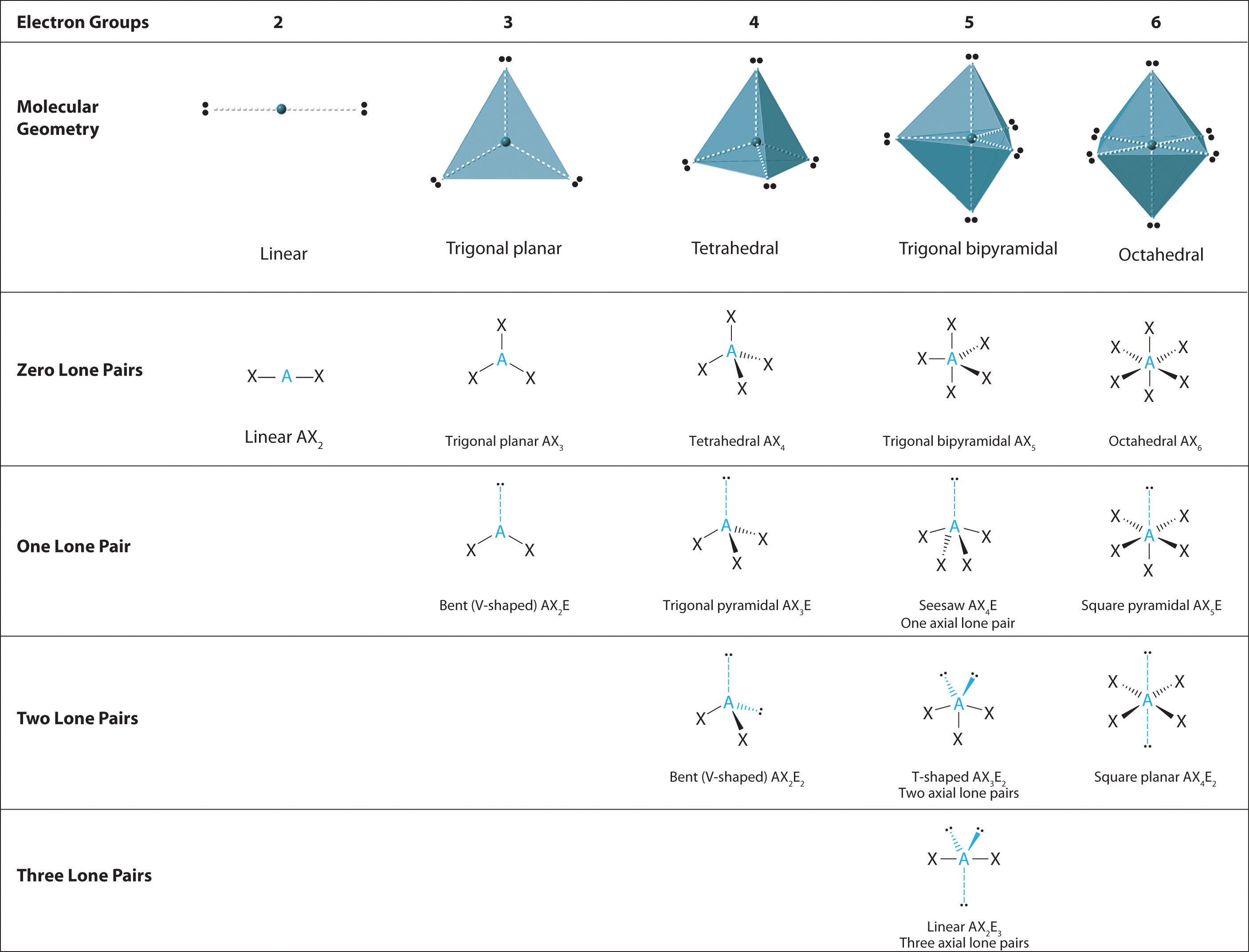
9.7 The Shapes of Molecules Chemistry LibreTexts
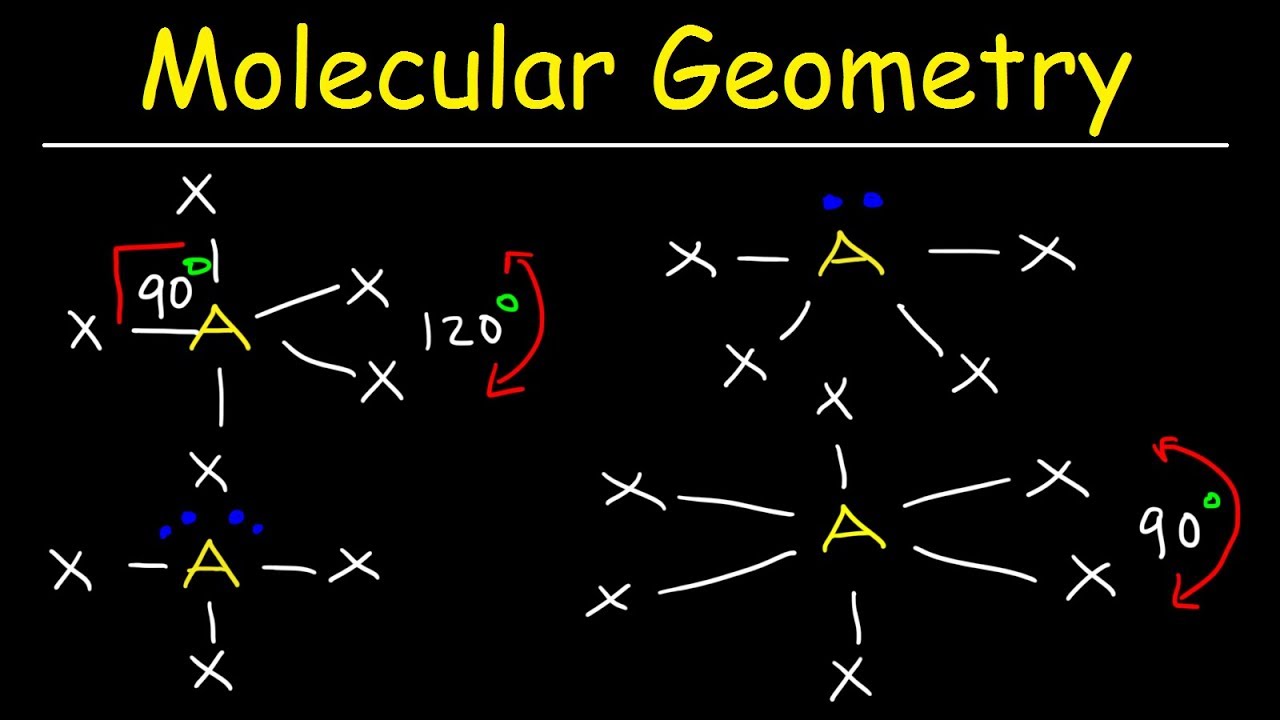
Molecular Geometry & VSEPR Theory Basic Introduction YouTube
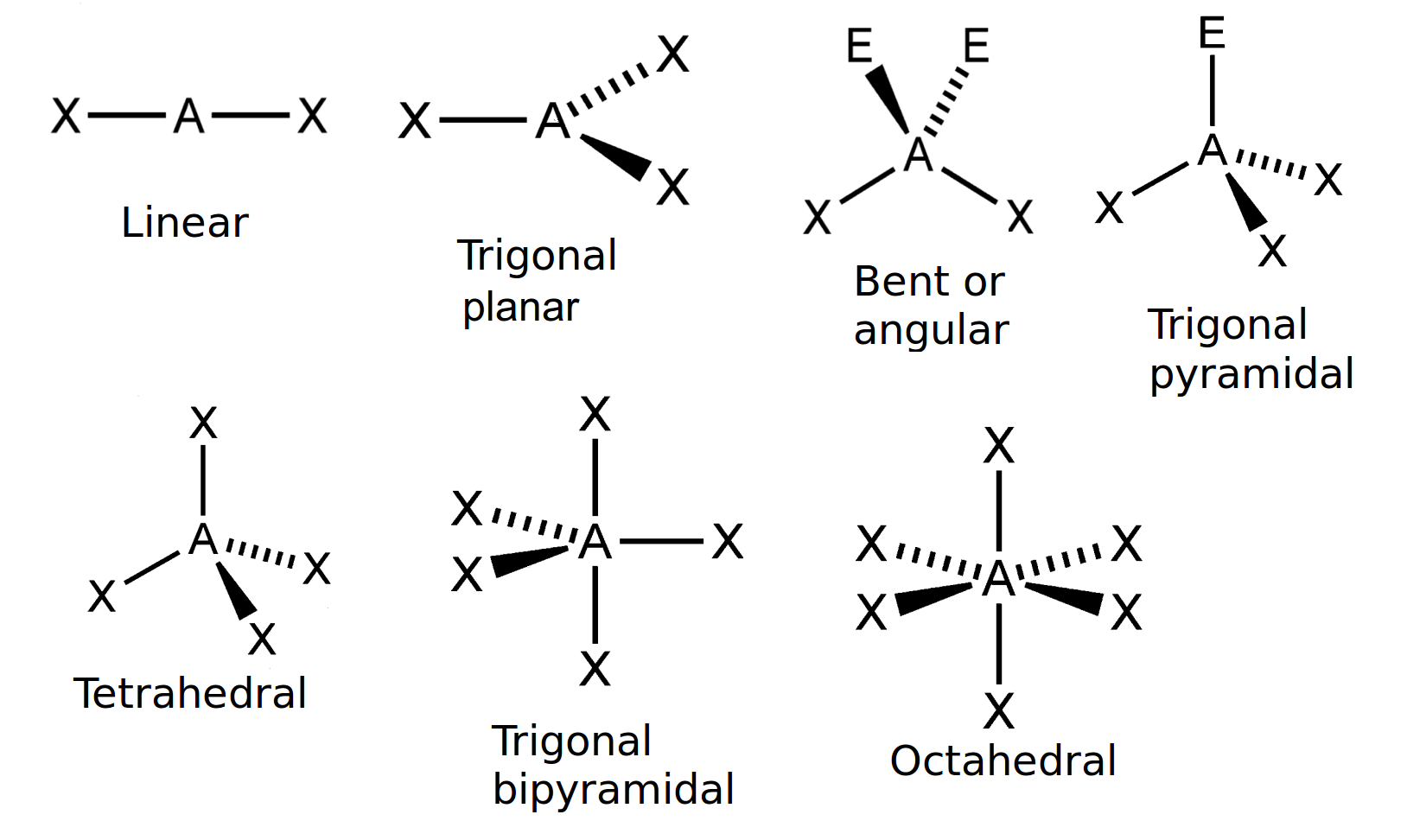
3.2 Molecular shape Atomic combinations Siyavula
The Net Dipole Is The Measurable, Which Is Called The Dipole Moment.
Μ = Δ × D (3.7.1) (3.7.1) Μ = Δ × D.
The Second Figure Serves As A Visual Aid For The Table.
A Table Of Geometries Using The Vsepr Theory Can Facilitate Drawing And Understanding Molecules.
Related Post: