How To Draw Compound
How To Draw Compound - Select the central atom which is least electronegative. Web we recommend you use a larger device to draw your structure. You will also find examples, exercises, and tips on how to avoid common mistakes. Step 2 tells how many electrons are needed and step 1 is how many electrons you have. Determine how many electrons must be added to the central element. Place one electron pair between each pair of adjacent atoms (as determined from the framework found in step 2) to form a single bond. The example is for the nitrate ion. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Press through your feet and send your hips back. Covalent bonds are shown using lines. The resulting smiles or inchi string may be used to search for matching molecules in the pdb chemical component dictionary. Each nitrogen atom has three bonds. Web bend your knees and keep your back straight. Let’s draw the lewis structure of hydrogen chloride, or hydrochloric acid in an aqueous solution. Covalent bonds are shown using lines. The example is for the nitrate ion. Web that’s why we’ve created this fun set of compound word read & draw worksheets. Covalent bonds are formed when one electron from each atom forms an electron pair. Each carbon atom has four bonds. Each oxygen atom has two bonds. It also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. Web here are the steps to draw a lewis structure. Drawing lewis structures of covalent molecules is shared under a not declared license and was authored, remixed, and/or curated by libretexts. On completion of the editing of the structure you can. Web place least electronegative element in center and draw single bonds from the central atom to other atoms. Covalent bonds are shown using lines. A structural formula displays the atoms of the molecule in the order they are bonded. Web how to draw constitutional isomers (structural isomers) and stereoisomers. Grip the bar on either side of you, brace your core. Kekulé formulas or structural formulas display the atoms of the molecule in the order they are bonded. Press through your feet and send your hips back. First thing first, don't trust height charts! To use, students will read each compound word aloud, then draw and color a picture to match. Shared pairs of electrons are drawn as lines between atoms,. Web properly drawing a compound bow is safe and efficient. Same skeleton (including h) same skeleton (excluding h) Web to achieve stability, it would react with another element, either by sharing electrons in a covalent bond or receiving electrons in an ionic bond. Note that matches will include any chemical component in the dictionary, including polymeric ones like alanine or. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Kekulé formulas or structural formulas display the atoms of the molecule in the order they are bonded. Drawing lewis structures. Web steps for writing lewis structures. Web here are the steps to draw a lewis structure. Web to achieve stability, it would react with another element, either by sharing electrons in a covalent bond or receiving electrons in an ionic bond. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Subtracting the. Each nitrogen atom has three bonds. Place one electron pair between each pair of adjacent atoms (as determined from the framework found in step 2) to form a single bond. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. Each h atom (group 1) has. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Each carbon atom has four bonds. Hydrogen and fluorine can never. The example is for the nitrate ion. Web place least electronegative element in center and draw single bonds from the central atom to other atoms. Lexi keller, with the help of mike schloesser, explains how to keep your compound draw on point.subscr. You will also find examples, exercises, and tips on how to avoid common mistakes. Press through your feet and send your hips back. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. He wanted to record iphone video of. Let’s draw the lewis structure of hydrogen chloride, or hydrochloric acid in an aqueous solution. Select the central atom which is least electronegative. Web here are the steps to draw a lewis structure. Web the best way to explain how to draw an isomer is to use an example. Each nitrogen atom has three bonds. Grip the bar on either side of you, brace your core and set your shoulders back and down. Hydrogen and fluorine can never. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Covalent bonds are shown using lines. In this case, we’re going to use c 6 h 14.
Cute How To Draw A Compound In Marvin Sketch Sketch Art Drawing

3 Ways to Draw Lewis Dot Structures wikiHow
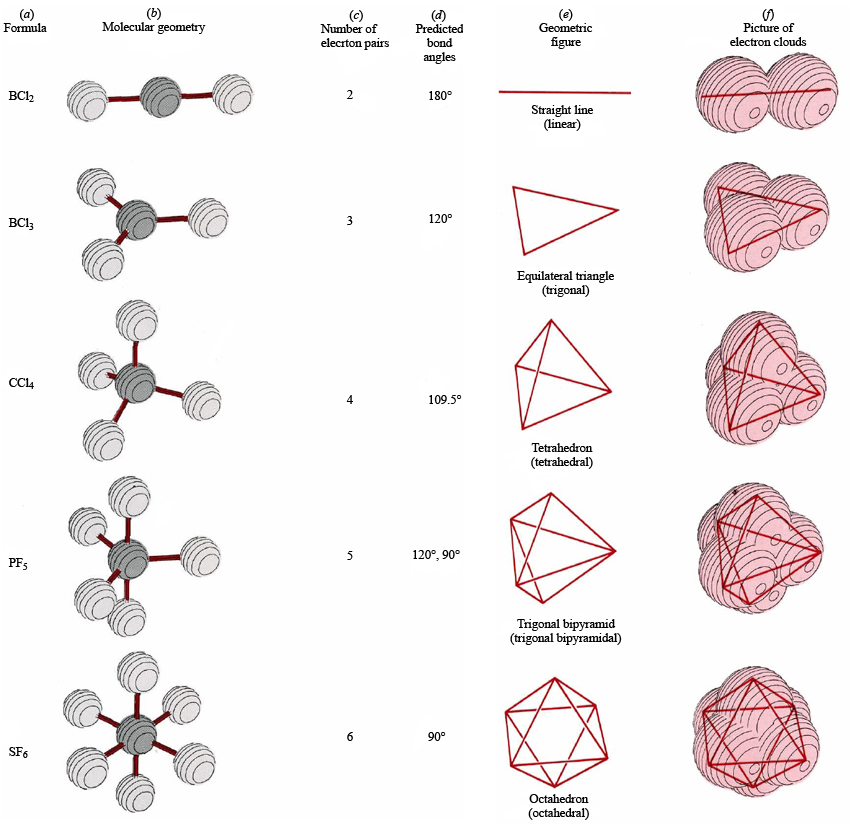
How can I find the molecular geometry of a compound? Socratic

How to draw a compound bow Archery 360 YouTube
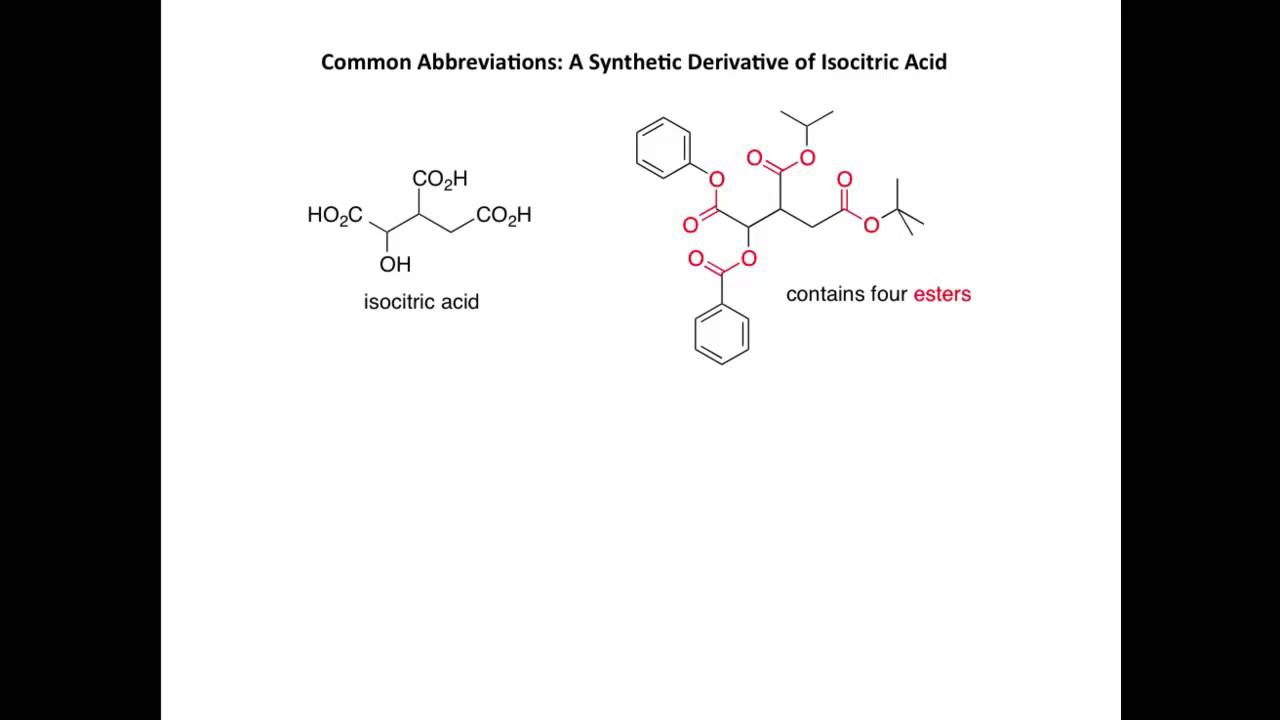
Drawing organic compounds YouTube
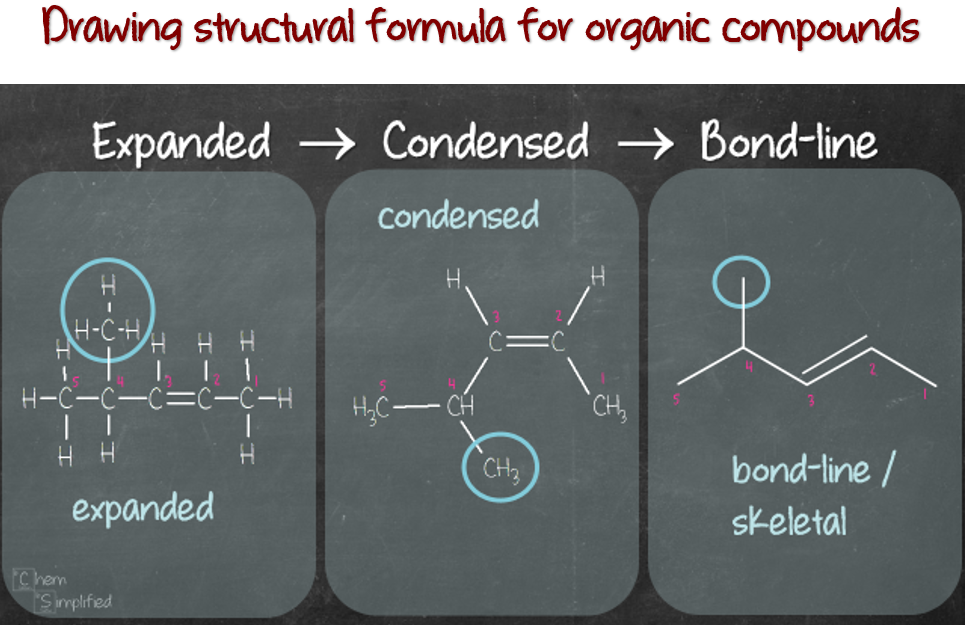
Organic Chemistry 101 Drawing the structures ChemSimplified

Example of chem draw traderpsawe
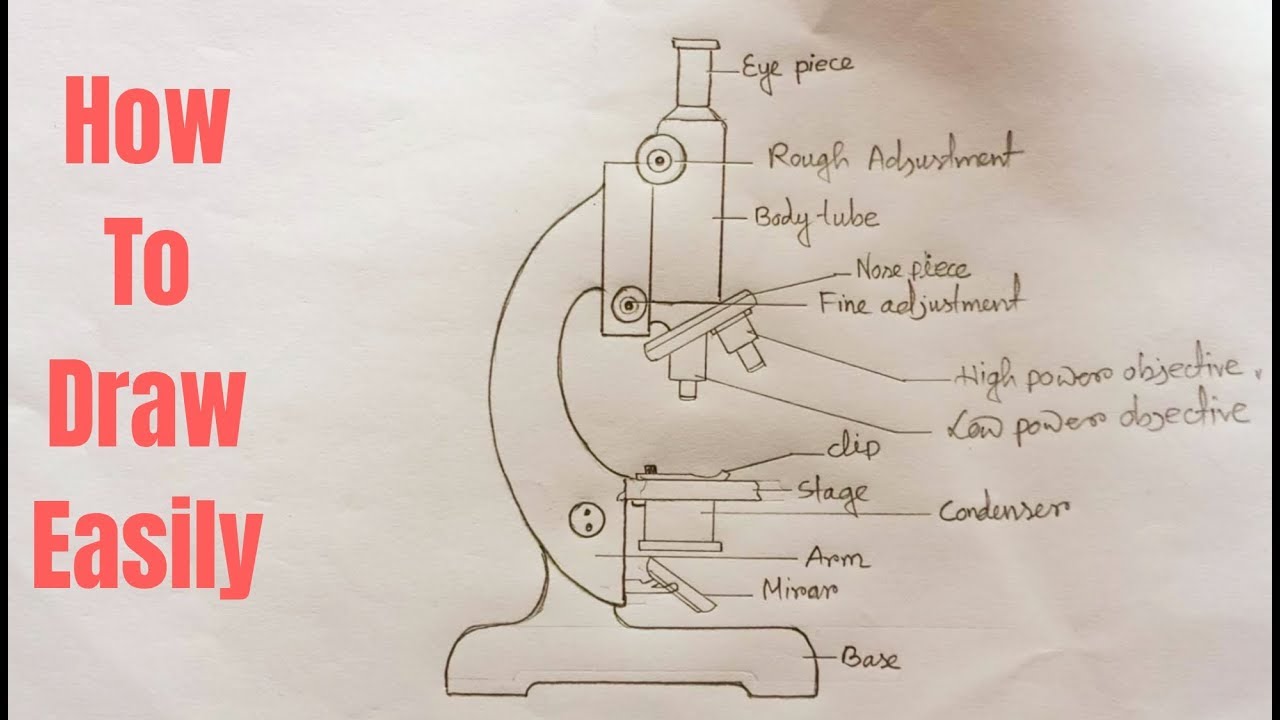
How to draw compound of Microscope easily step by step YouTube

Chemsketch tutorial (how to draw chemical structures, Lecture 1) YouTube
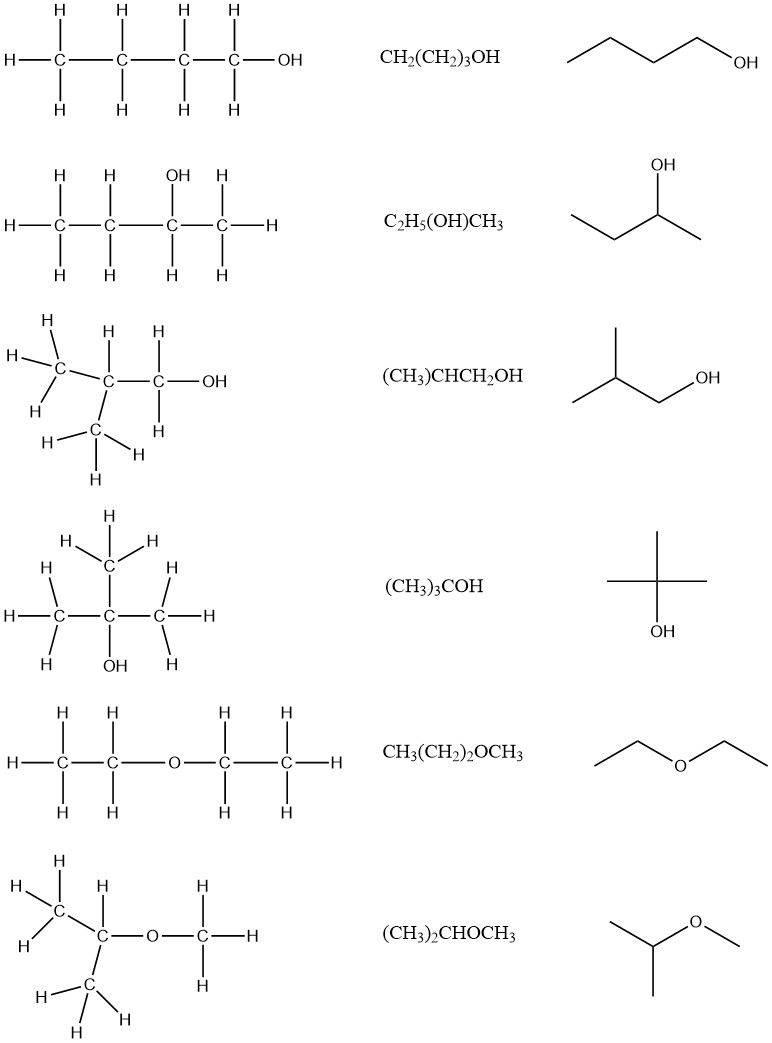
1.13 Drawing Chemical Structures Chemistry LibreTexts
First Thing First, Don't Trust Height Charts!
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
Web For Anions Add A Number Of Electrons Equal To The Negative Charge.
When Drawing The Structure Of A Neutral Organic Compound, You Will Find It Helpful To Remember That.
Related Post: