How To Draw Bond Dipoles
How To Draw Bond Dipoles - Web the organic chemistry tutor. The other atom involved in the bond, the one with the lower electronegativity, would have a partial positive charge, with the plus part of the arrow. Web after drawing a lewis structure, you'd draw a dipole arrow pointing towards the atom with higher electronegativity. This organic chemistry video explains how to determine if a molecule is polar and has net dipole moment. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. Bcl 3, for example, has no dipole moment, while nh 3 does. The difference in electronegativity can be used to determine. A small plus sign is drawn on the less electronegative end to indicate the partially positive end of the bond. Web subtract the two values, and point the negative end of the bond dipole toward the atom of greater electronegativity. If a substance is both a hydrogen donor and a hydrogen bond acceptor, draw a structure showing the hydrogen bonding. Just place a substance between charged plates (figure \(\pageindex{2}\)); The difference in electronegativity can be used to determine. And in this case we have four dipoles, but they're going to cancel out in three dimensions. If a substance is both a hydrogen donor and a hydrogen bond acceptor, draw a structure showing the hydrogen bonding. Web learn about carbon dioxide's. Dipole moments arise from differences in electronegativity. Web the convention in chemistry is that the arrow representing the dipole moment goes from positive to negative. 106k views 1 year ago. We start by looking at a water molecule: The dipole moment of a molecule and its overall polarity depends on the magnitude and direction of individual polar bonds and their. 106k views 1 year ago. Example between o and f, the dipole would point to f. A dipole arrow is crossed at the beginning (as in a plus sign) and points in the direction of the greatest electron density. If they have a net dipole moment then the molecule is polar. Web the dipole should point to the more electronegative. Example between o and f, the dipole would point to f. Physicist tend to use the opposite orientation. It is relatively easy to measure dipole moments: Using the cross bow arrow shown below we can show that it has a net dipole. This organic chemistry video tutorial provides a basic introduction into. We can draw four of them here. Example between o and f, the dipole would point to f. Web the convention in chemistry is that the arrow representing the dipole moment goes from positive to negative. This is because this atom attract electrons and gains a partial positive charge. From in between the hydrogen atoms to the oxygen atom. Web the dipole moment points in the direction of the vector quantity of each of the bond electronegativities added together. The dipole moment of a molecule and its overall polarity depends on the magnitude and direction of individual polar bonds and their dipole moments. Conversely, the presence or absence of a dipole moment may also give an important clue to. A dipole arrow is crossed at the beginning (as in a plus sign) and points in the direction of the greatest electron density. This organic chemistry video explains how to determine if a molecule is polar and has net dipole moment. Physicist tend to use the opposite orientation. This organic chemistry video tutorial provides a basic introduction into. It explains. Conversely, the presence or absence of a dipole moment may also give an important clue to a compound’s structure. This organic chemistry video explains how to determine if a molecule is polar and has net dipole moment. Example between o and f, the dipole would point to f. This organic chemistry video tutorial provides a basic introduction into. The bond. A dipole arrow is crossed at the beginning (as in a plus sign) and points in the direction of the greatest electron density. Web dipole vectors are shown as arrows pointing along the bond from the less electronegative atom toward the more electronegative atom. We start by looking at a water molecule: Web the convention in chemistry is that the. The difference in electronegativity can be used to determine. 106k views 1 year ago. From in between the hydrogen atoms to the oxygen atom. The bond dipole moment that arises in a chemical bond between two atoms of different electronegativities can be expressed as follows: Example between o and f, the dipole would point to f. You must be able to combine your knowledge of molecular shapes and bond polarities to determine whether or not a given compound will have a dipole moment. This organic chemistry video tutorial provides a basic introduction into. If they have a net dipole moment then the molecule is polar. Web this chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond. Example between o and f, the dipole would point to f. Looking at the electronegativity and shape of the h2o molecule tells you how the arrow depicts the polarity: From in between the hydrogen atoms to the oxygen atom. Bcl 3, for example, has no dipole moment, while nh 3 does. Web the magnitude of a bond dipole moment is represented by the greek letter mu ( µ) and is given by the formula shown here, where q is the magnitude of the partial charges (determined by the electronegativity difference) and r. The vector points from positive to negative, on both the molecular (net) dipole moment and. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket press copyright. The other atom involved in the bond, the one with the lower electronegativity, would have a partial positive charge, with the plus part of the arrow. Web the organic chemistry tutor. Web after drawing a lewis structure, you'd draw a dipole arrow pointing towards the atom with higher electronegativity. The larger the difference in electronegativity, the larger the dipole moment. If you require something more accurate, you should be able to look up the dipole moments for a variety of bonds.[Solved] How do I draw dipole image that has the net dipole vectors and
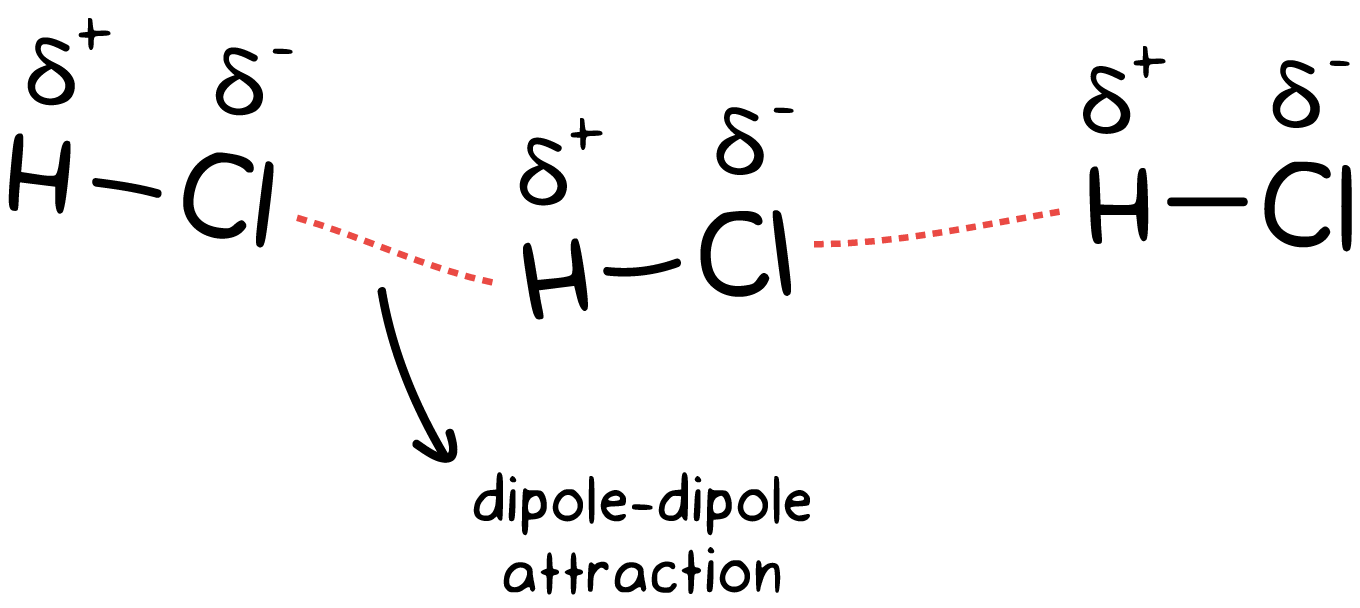
Intermolecular Force Types and Examples StudiousGuy

Molecular Polarity Molecular Structure ppt video online download

Dipole Dipole Forces of Attraction Intermolecular Forces YouTube

How To Draw Overall Dipole Moment DRAWINGS OF LOVE
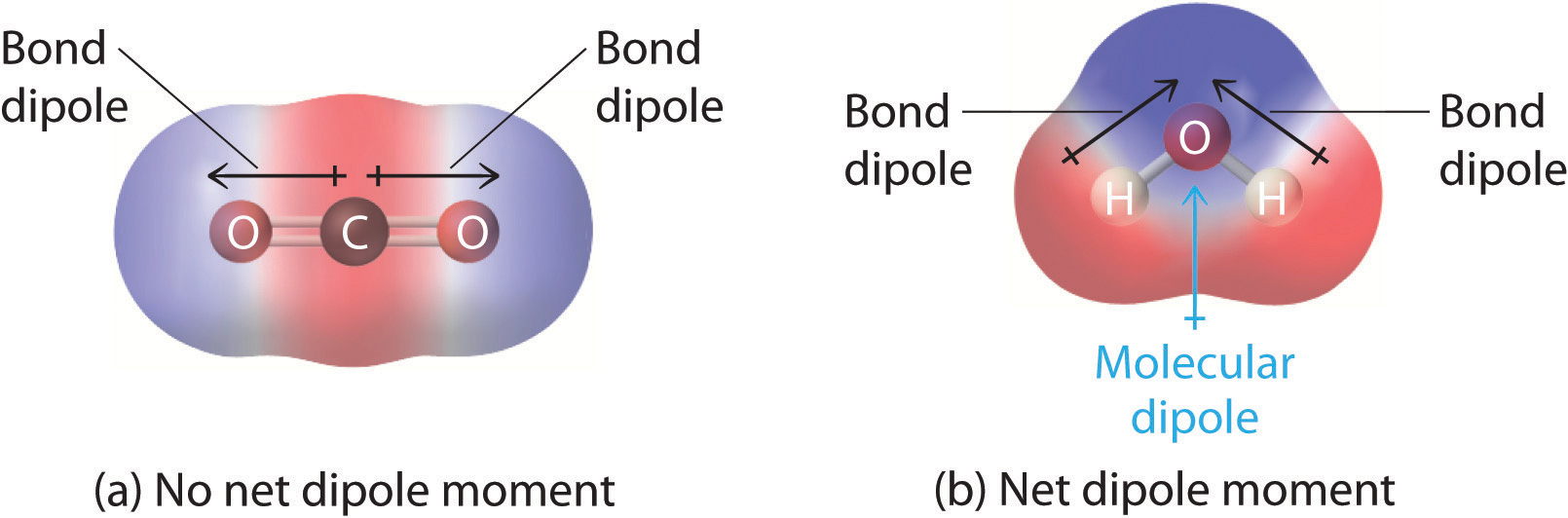
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts

Lewis Structure NH3 plus dipoles, shape, angles and formal charge
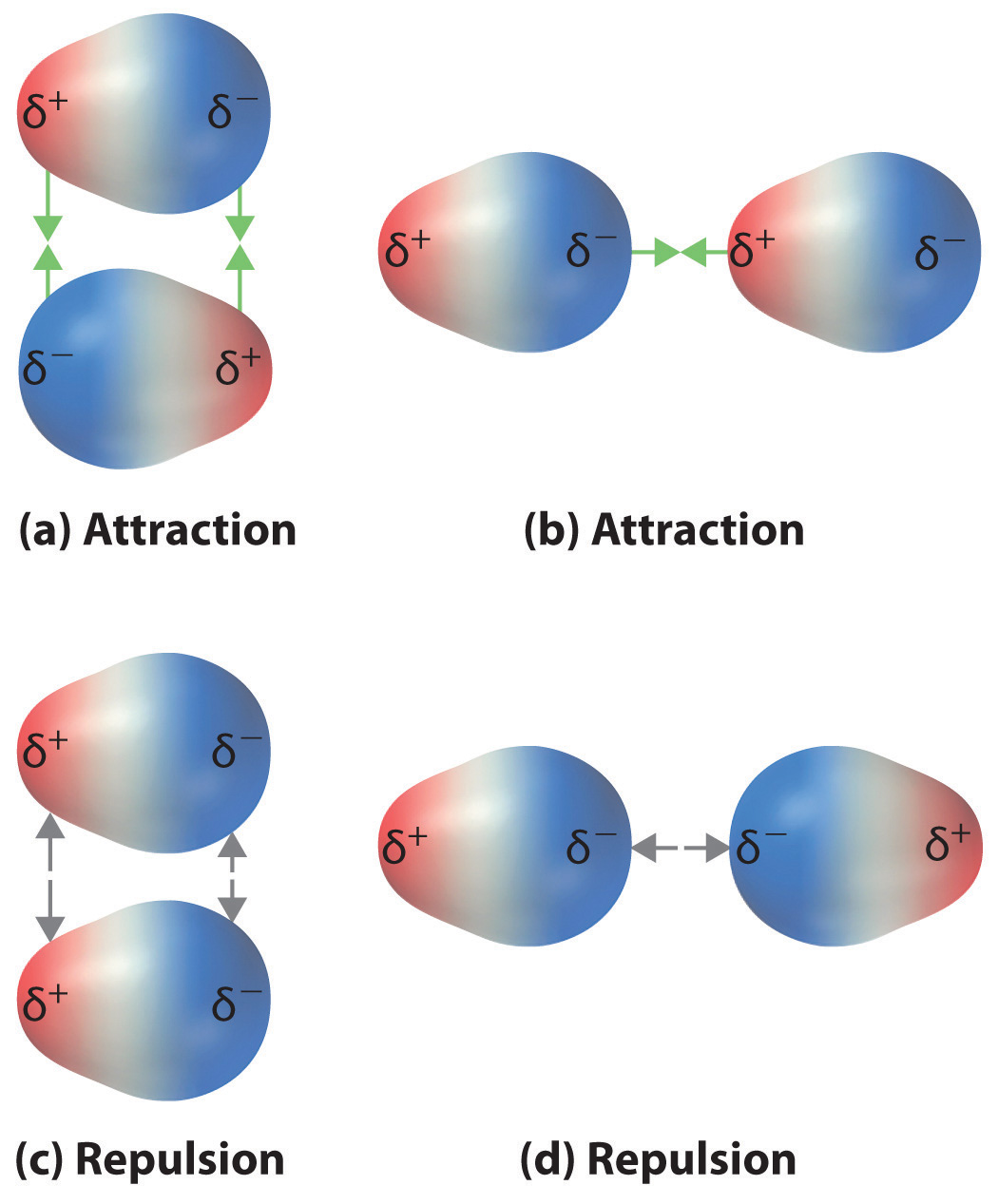
CH103 Chapter 5 Covalent Bonds and Introduction to Organic Molecules

How To Draw Overall Dipole Moment DRAWINGS OF LOVE

Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps
Web Dipole Vectors Are Shown As Arrows Pointing Along The Bond From The Less Electronegative Atom Toward The More Electronegative Atom.
Web The Convention In Chemistry Is That The Arrow Representing The Dipole Moment Goes From Positive To Negative.
And In This Case We Have Four Dipoles, But They're Going To Cancel Out In Three Dimensions.
Web The Dipole Moment Points In The Direction Of The Vector Quantity Of Each Of The Bond Electronegativities Added Together.
Related Post: