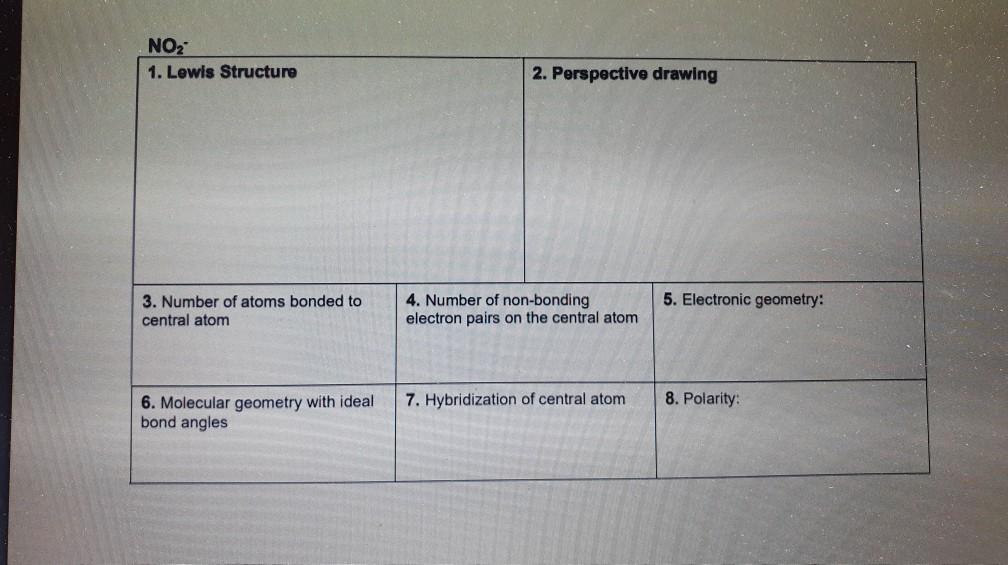
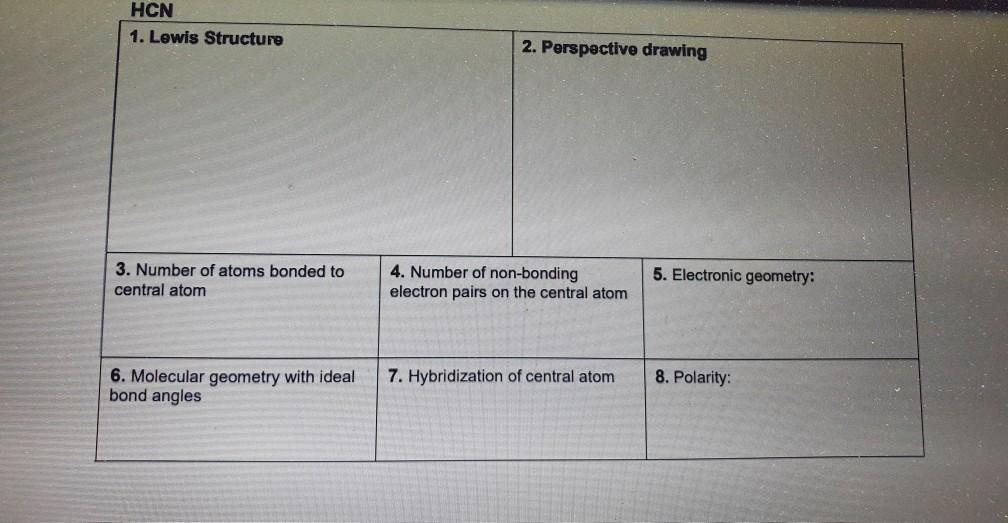
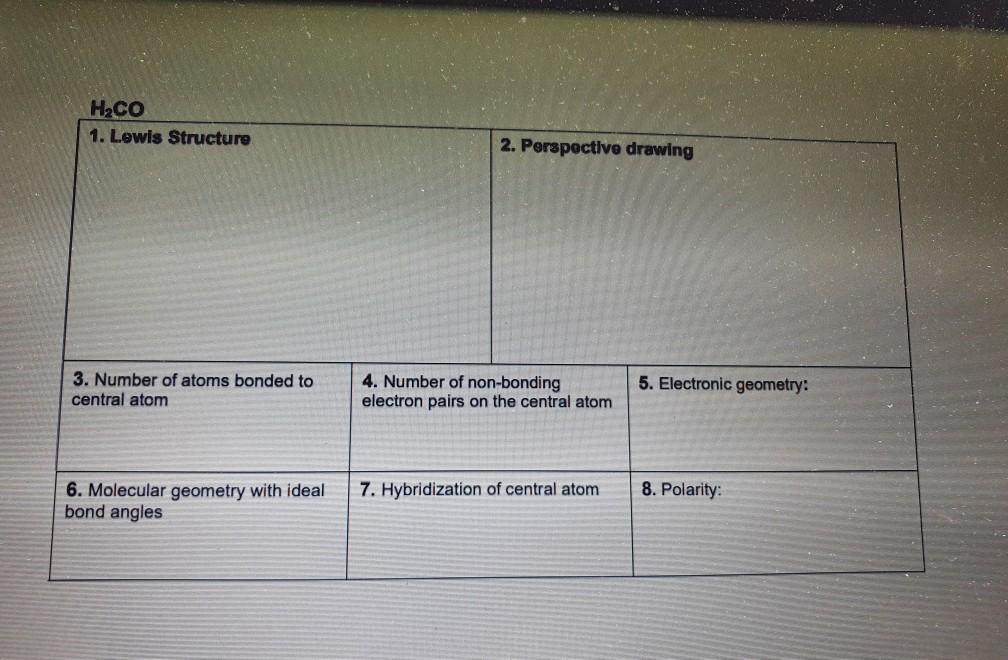
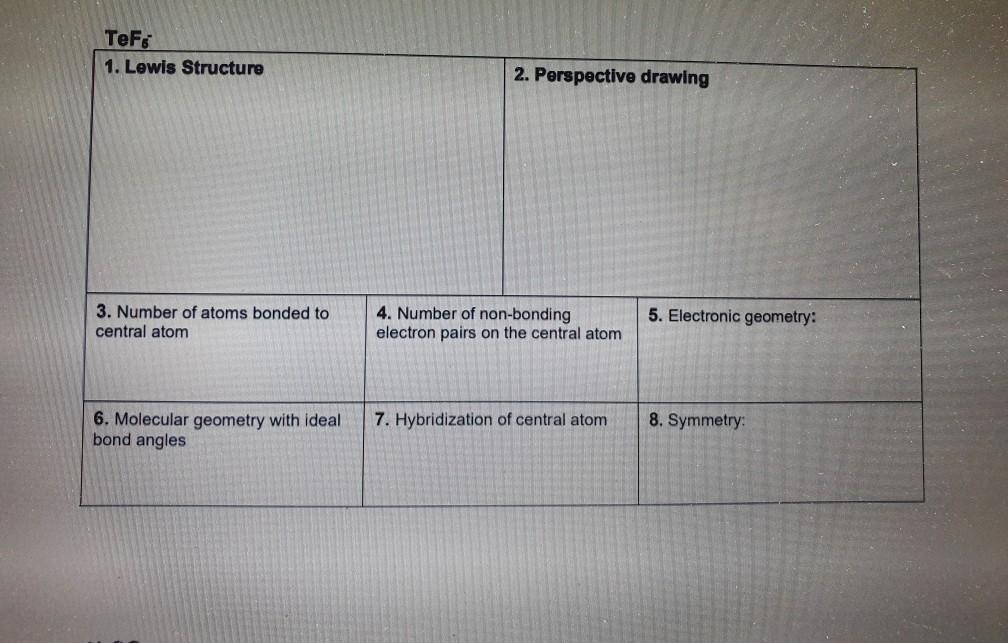
Hcn Perspective Drawing
Hcn Perspective Drawing - 14k views 10 years ago. 925 views 2 years ago playlist:lewis structure (click here to learn more) how do you draw the lewis structure of hcn (hydrogen cyanide)? Number of atoms bonded to central atom 4. # of atoms bonded to 5. Web 6 steps to draw the lewis structure of hcn. The compound has sp hybridization. Here is a little flow chart of how we are going to do this: Average rating 3.4 / 5. #3 calculate and mark formal charges on the atoms, if required. Hybridization of central atom 8. Here is a little flow chart of how we are going to do this: Use these steps to correctly draw the hcn lewis structure: For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. 14k views 10 years ago. Web 6 steps to draw the lewis structure of hcn. Web draw lewis structures, including all resonance structures if applicable (1). With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms. H + c + n =1 + 4 + 5 = 10. He was obsessed with the vanishing point, and also birds (cute!). Electronic geometry central. Here, the given molecule is hcn. Number of atoms bonded to central atom 4. Build a variety of molecules and ions using molecular model kits. Using formal charges to determine how many bonds to make, a different perspective. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Here is a little flow chart of how we are going to do this: Determine the hybridization of the central atoms, the number and types of bonds, the geometries, and the polarities of the molecules and ions. He was obsessed with the vanishing point, and also birds (cute!). #1 first draw a rough sketch. The compound is polar in nature. Web to draw lewis structures. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. #of atoms bonded to 5. Steric number of central 4. The compound has sp hybridization. He was obsessed with the vanishing point, and also birds (cute!). H + c + n =1 + 4 + 5 = 10. Calculate the total number of valence electrons. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Web 6 steps to draw the lewis structure of hcn. The compound is polar in nature. Build models and then draw perspective structures (2) that accurately represent bond angles and molecular shapes. Find the total valence electrons in hcn molecule. Calculate the total number of valence electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web drawing lewis structures for molecules with one central atom: Bonding and polarity data table 2 molecule perspective drawing electronegativity bond dipole polarity polarity moment becl2 ch nh, h.o sncl hcn if. Use these steps to correctly draw the hcn lewis structure: Put the least electronegative atom c in the middle with h and cl on either side. Add these. Molecular geometry with ideal bond angles 7. #of atoms bonded to 5. Here's how to do it. Here is a little flow chart of how we are going to do this: Hybridization of central atom 8. Here's how to do it. Molecular geometry with ideal bond angles 7. Steric number of central 4. Calculate the total number of valence electrons. Chemical engineering questions and answers. Make sure you put the correct atom at the center of the hcn molecule. Molecular geometry with ideal bond angles 7. In order to draw the lewis structure of hcn, first of all you have to find the total number of valence electrons present in the hcn molecule. Count the valence electrons you can use. 925 views 2 years ago playlist:lewis structure (click here to learn more) how do you draw the lewis structure of hcn (hydrogen cyanide)? Use these steps to correctly draw the hcn lewis structure: #2 mark lone pairs on the atoms. Web click on a star to rate it! Step method to draw the lewis structure of hcn. Put the least electronegative atom c in the middle with h and cl on either side. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. Build models and then draw perspective structures (2) that accurately represent bond angles and molecular shapes. The compound is polar in nature. Determine the hybridization of the central atoms, the number and types of bonds, the geometries, and the polarities of the molecules and ions. Electronic geometry central atom atom 7. Molecular geometry with ideal bond angles 7.Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
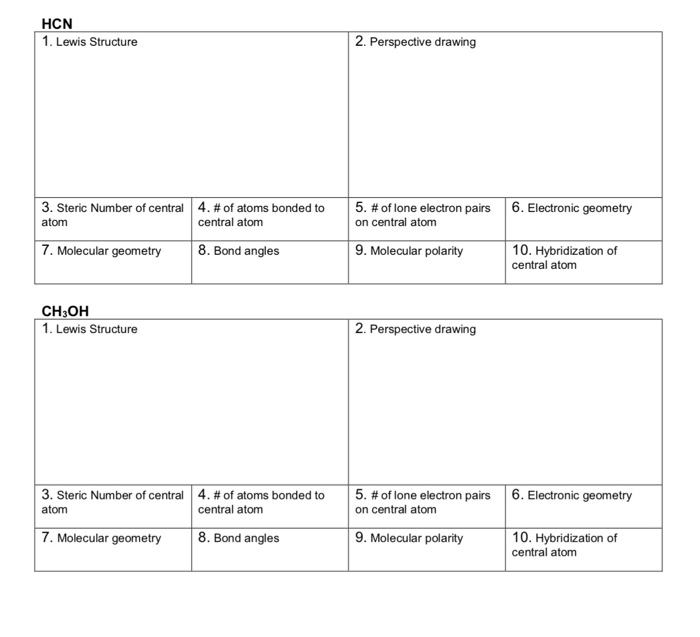
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
Solved HCN 1. Lewis Structure 2. Perspective drawing 3.
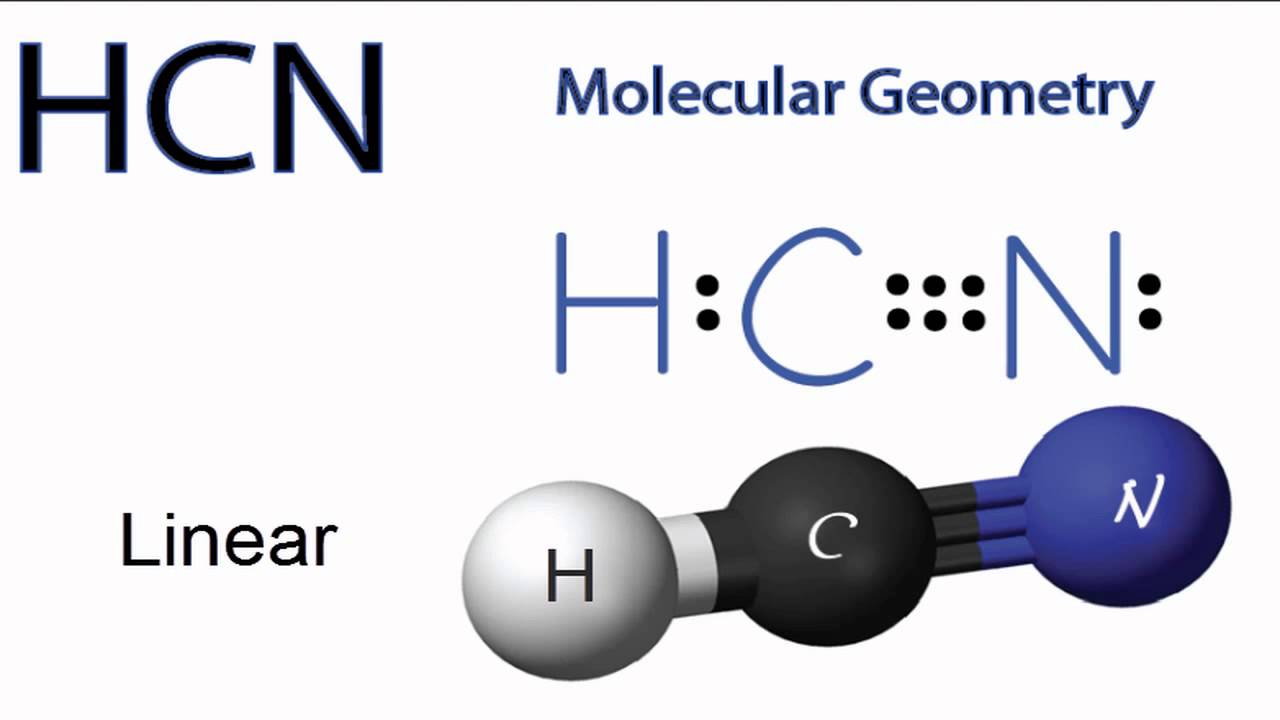
HCN Molecular Geometry YouTube

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and

Lewis Dot Diagram Of Hcn
Be Sure That You Don't Use More Than The Ten Valence Electrons Available.
Calculate The Total Number Of Valence Electrons.
Web Hcn Lewis Structure And Molecular Geometry Step By Step.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Related Post: