H2O Drawing
H2O Drawing - #3 if needed, mention formal charges on the atoms. Web we draw lewis structures to predict: Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. Drawing the lewis structure for h 2 o. * hydrogen atoms are always terminal (only one bond) Place the remaining electrons on the atoms; O has 6 valence electrons, and each h has one. Determine the total number of valence electrons; When you are learning to draw lewis structures you will see this one frequently. The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. Web the h2o lewis structure shows the two hydrogen atoms bonded to the oxygen atom, and the two unshared electron pairs on. How to draw lewis structure for h 2 o. All of the electron pairs—shared and unshared—repel each other. Place the remaining electrons on the atoms; Valence electrons are the electrons in the outermost shell of an atom. Web bi purchased a container with a spigot to dispense water in a controlled fashion, first at 50 degrees fahrenheit, then at 194. Count the total number of valence electrons: Connect the atoms with single bonds; To determine the total number of valence electrons in h2o, add up the valence electrons of each atom. See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. Drawing upon the finding, the way towards improving. #2 mention lone pairs on the atoms. Web drawing the lewis structure of water (h2o) involves representing the valence electrons of the hydrogen and oxygen atoms and arranging them to form covalent bonds between the atoms. Drawing the lewis structure for water. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ).. You can find a procedure for drawing lewis structures at this location. Web what is the lewis structure of water h2o? How to draw lewis structure for h 2 o. All of the electron pairs—shared and unshared—repel each other. Water molecule is a simple molecule. How to draw the h2o lewis structure. Find the point group of the molecule and assign cartesian coordinates so that z is the principal axis. Web drawing the lewis structure of water (h2o) involves representing the valence electrons of the hydrogen and oxygen atoms and arranging them to form covalent bonds between the atoms. Web atoms can form more than. Molecular geometry of h 2 o; I quickly take you through how to draw the lewis structure of water, h2o. * hydrogen atoms are always terminal (only one bond) #2 mention lone pairs on the atoms. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web how to draw lewis structure for h 2 o; Web the h2o lewis structure shows the two hydrogen atoms bonded to the oxygen atom, and the two unshared electron pairs on the oxygen atom. Web what is the lewis structure of water h2o? Web explore the molecular structure and geometry of water, one of the most common and important. The structures of h 2, f 2, and h 2 o would usually be drawn as follows: What, how to balance &. Web the h2o lewis structure shows the two hydrogen atoms bonded to the oxygen atom, and the two unshared electron pairs on the oxygen atom. Chemists normally represent a bond using a line instead of two dots. Web. When you are learning to draw lewis structures you will see this one frequently. #2 mention lone pairs on the atoms. There is no need to simplify this problem, as we had done for previous examples. Count the total number of valence electrons: * hydrogen atoms are always terminal (only one bond) Valence electrons are the electrons in the outermost shell of an atom. Polarity of h 2 o; Molecular geometry of h 2 o; #3 if needed, mention formal charges on the atoms. It is eight to form a single h2o molecule. Web follow these steps to draw the lewis structure of h2o: See how the two hydrogen atoms and one oxygen atom are bonded together in this interactive 3d model. In short, these are the steps you need to follow for drawing a lewis structure: Hybridization of h 2 o; You can find a procedure for drawing lewis structures at this location. #1 draw a rough skeleton structure. Web what is the lewis structure of water h2o? O has 6 valence electrons, and each h has one. #2 mention lone pairs on the atoms. The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms. Web here, we need to understand how the lewis structure is drawn for the h2o molecule:
How To Draw Water Drops Really Easy Drawing Tutorial Water Drawing
How To Draw A Water Drop Water Drops Drawings Drawing Tutorial Images

H2O by LocoTheDrawfag on DeviantArt
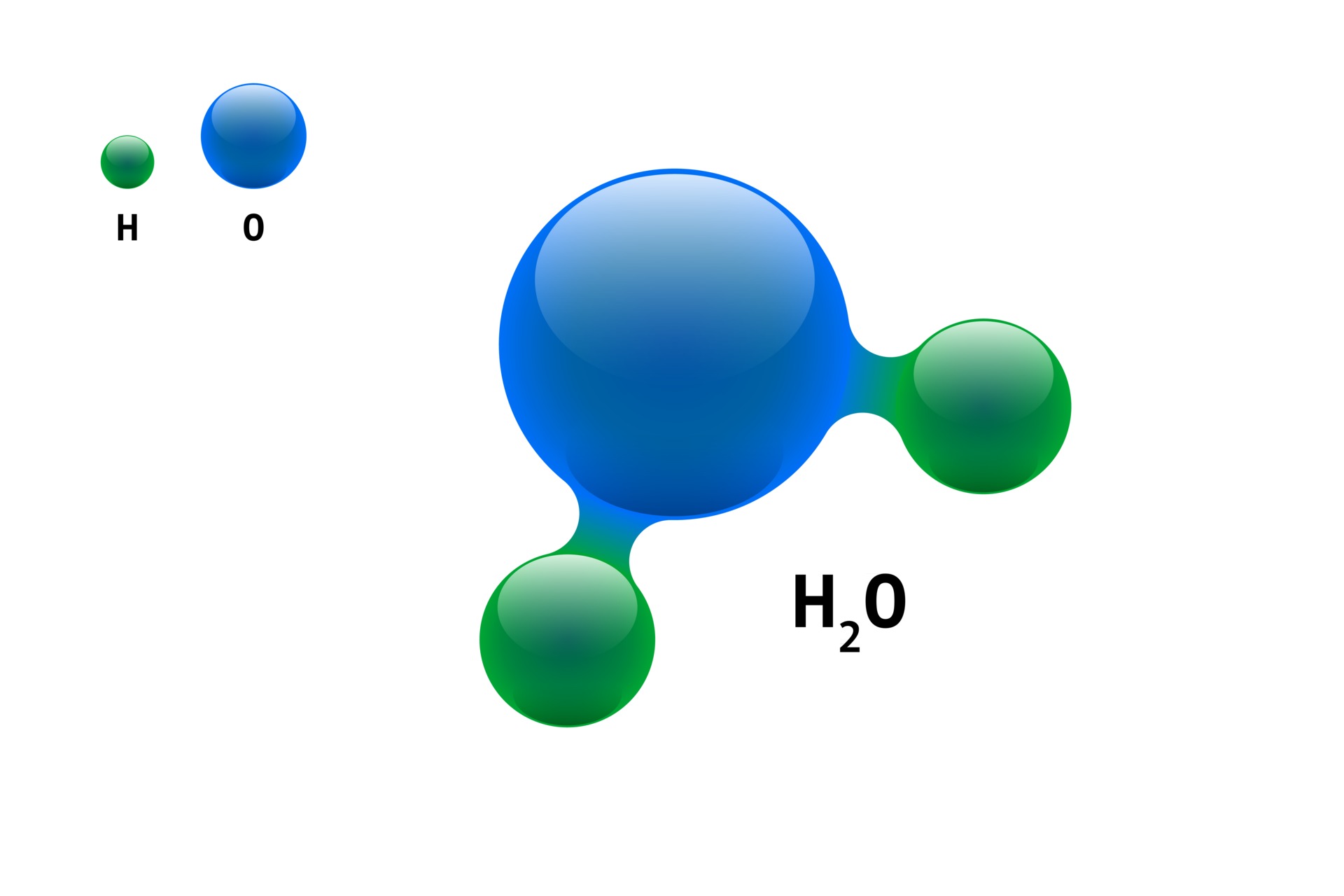
H2O Molecule Model

H2O Lewis Structure, Molecular Geometry, and Hybridization
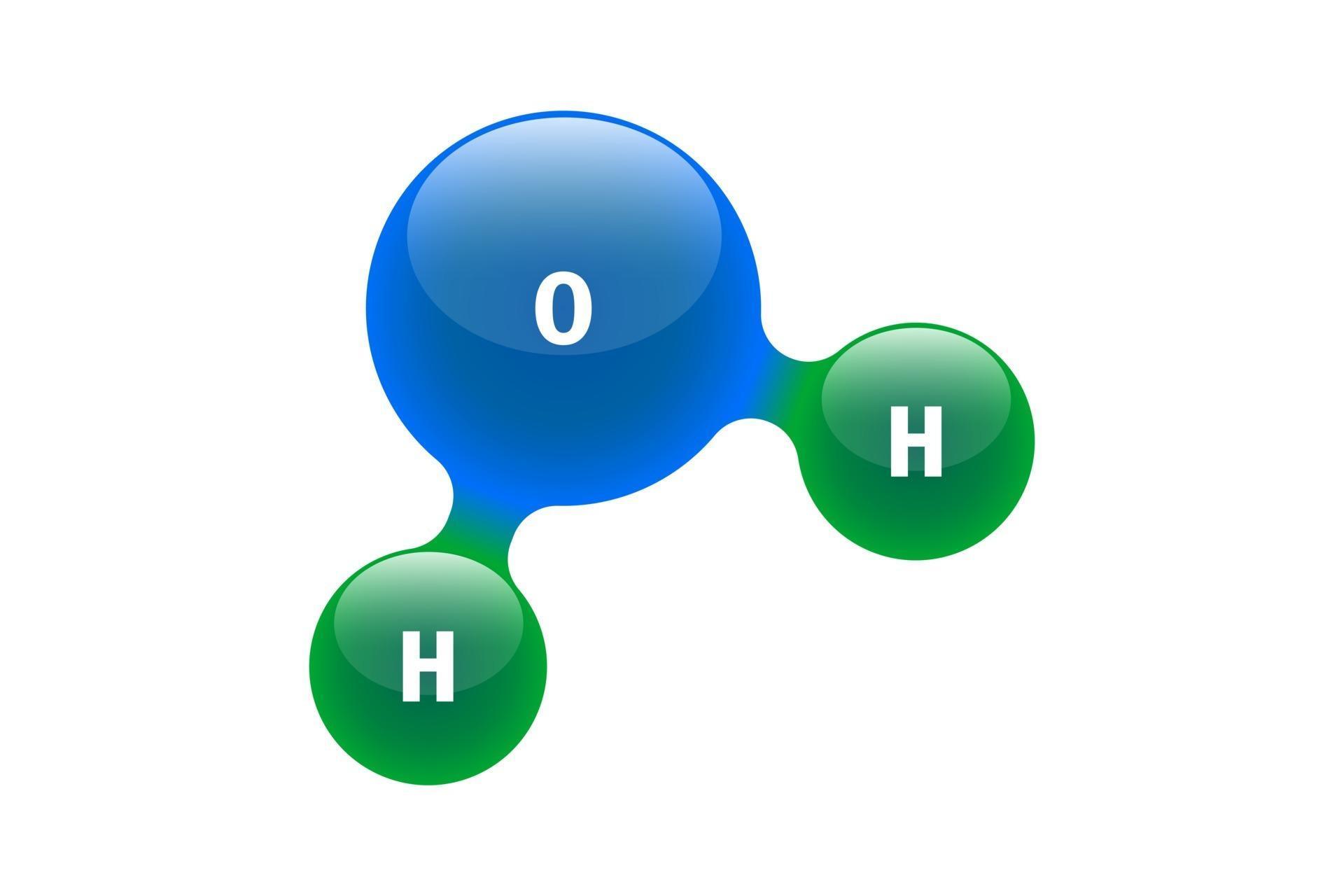
Chemistry model of molecule water H2O scientific elements. Integrated

H2O Royalty Free Vector Image VectorStock
H2o Drawing at Explore collection of H2o Drawing

Bottle of H2O by Colleen Sweeney Hand art kids, Bottle drawing, Save
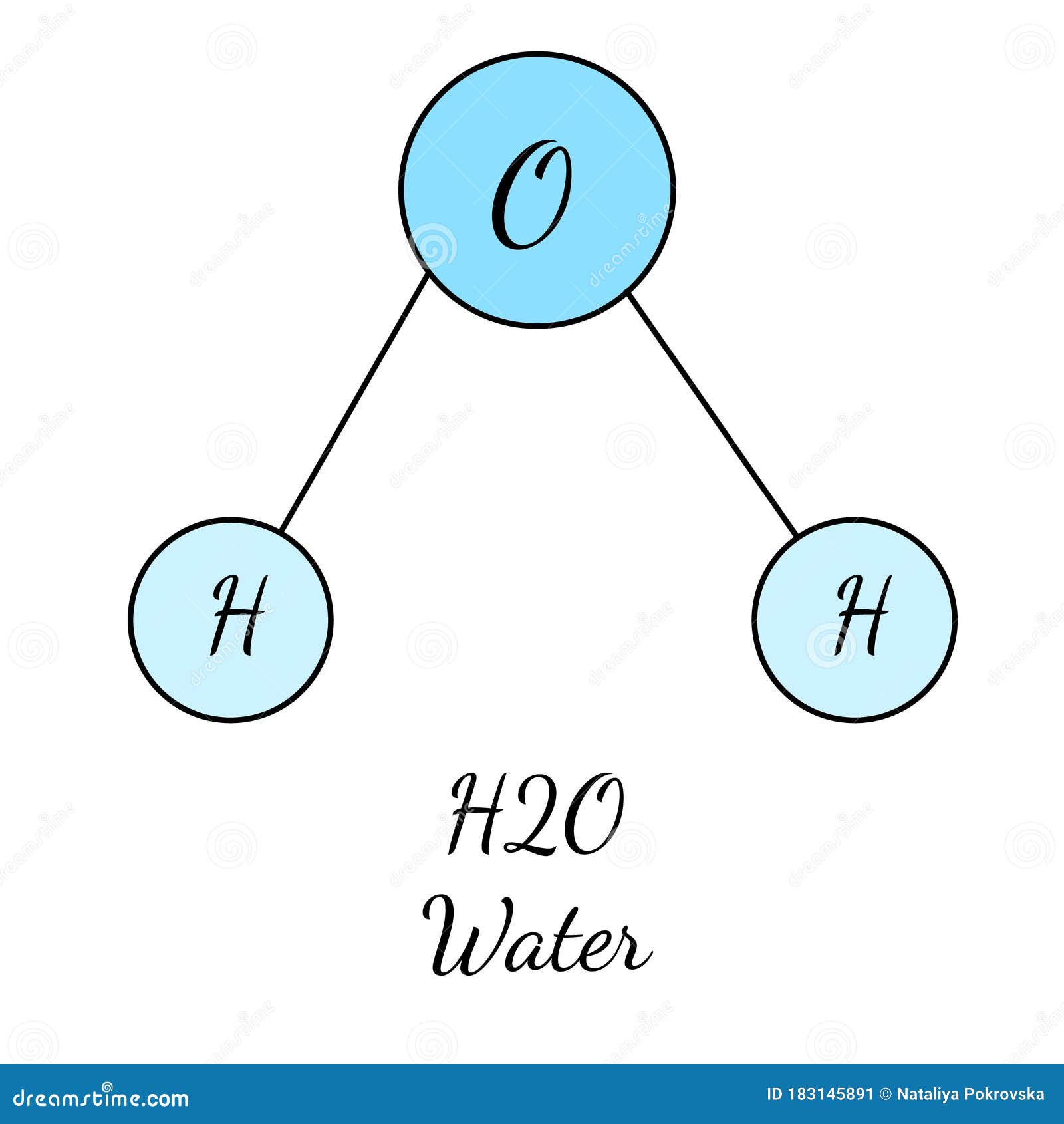
H2O Chemical Medical Formula For Water Molecula In Blue Color. Simple
Web The H2O Lewis Structure Shows The Two Hydrogen Atoms Bonded To The Oxygen Atom, And The Two Unshared Electron Pairs On The Oxygen Atom.
How To Draw The H2O Lewis Structure.
Look For How Many Electrons Are Needed:
Web Each Step Of Drawing Lewis Structure Of H 2 O Are Explained In This Tutorial.
Related Post: