Gspr Checklist Template
Gspr Checklist Template - Web mdrg has created a general safety & performance requirements checklist that contains a full table of the requirements, along with a list of applicable standards. Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements as well as a list of. (just in case if no one shares any template here) actually it is not difficult to create your own checklist. Web in the gspr checklist clearly mention the below points: Web every single section of the eu mdr or ivdr gspr should be assessed in its own right, as it pertains to your medical device. The completed checklist must include: It is planned that these templates will be withdrawn once the eudamed module for clinical. Web what should you include in your gspr checklist? “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. Mention the international standard (preferably harmonized) or the common specification (cs) applicable. Web what should you include in your gspr checklist? The general safety and performance requirements that apply to the device and an. When a requirement applies, a simple statement can. Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list into the articles, while other topics are new to the requirements list,. Web 4easyreg. The completed checklist must include: Web 4easyreg has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and performance requirements. “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. Web designed to support your conformity to annex i. Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements as well as a list of. The completed checklist must include: Mention the international standard (preferably harmonized) or the common specification (cs) applicable. “reduce as far as possible and appropriate the risks from unintended cuts. The completed checklist must include: Web what should you include in your gspr checklist? Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements as well as a list. The completed checklist must include: “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. Web trusted information resource. This excel spreadsheet is designed to support manufacturers making the transition from mdd to mdr / ivdd to ivdr. Web every single section of the eu mdr or ivdr gspr should be. The general safety and performance requirements that apply to the device and an. When a requirement applies, a simple statement can. Web 4easyreg has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and performance requirements. Mention the international standard (preferably harmonized) or the common specification (cs) applicable.. Web mdrg has created a general safety & performance requirements checklist that contains a full table of the requirements, along with a list of applicable standards. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. Web in the gspr checklist clearly mention the below points:. (just in case if no one shares any template here) actually it is not difficult to create your own checklist. The completed checklist must include: The general safety and performance requirements that apply to the device and an. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously. Web medical device regulation 2017/745 general essential safety and performance requirements check list template. It is planned that these templates will be withdrawn once the eudamed module for clinical. Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list into the articles, while other topics are new to the requirements list,. Web every single. Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list into the articles, while other topics are new to the requirements list,. (just in case if no one shares any template here) actually it is not difficult to create your own checklist. Web what should you include in your gspr checklist? When a requirement. This template acts as an invaluable tool in easing the. Web a library of free medical device templates and checklists for you to use to bring higher quality devices faster and continuously improve them. “reduce as far as possible and appropriate the risks from unintended cuts and pricks, such as needle stick injuries”. Web 4easyreg has made available a gspr checklist that will help you to ensure compliance of your devices and related documentation with the safety and performance requirements. When a requirement applies, a simple statement can. Web what should you include in your gspr checklist? Web designed to support your conformity to annex i of mdr 2017/745, this document contains a full table of mdr general safety and performance requirements as well as a list of. Web trusted information resource. It is planned that these templates will be withdrawn once the eudamed module for clinical. Web we offer an intuitive gspr checklist template, customised to accommodate your unique ivd medical devices. Web every single section of the eu mdr or ivdr gspr should be assessed in its own right, as it pertains to your medical device. Web planned to be conducted as to any specific national requirements. The general safety and performance requirements that apply to the device and an. The completed checklist must include: Web some topics such as clinical evaluation and medicinal consultation have moved from the requirements list into the articles, while other topics are new to the requirements list,. (just in case if no one shares any template here) actually it is not difficult to create your own checklist.
MDR 2017/745 GSPR template Easy Medical Device School

Mdr Gspr Checklist Template Printable Word Searches
![Free IVDR GSPR Checklist [Template] Trinzo](https://static.wixstatic.com/media/51879b_59a5cf39e38c4181a0b12fde3ab7cfb3~mv2.png/v1/fill/w_2240,h_1260,al_c/51879b_59a5cf39e38c4181a0b12fde3ab7cfb3~mv2.png)
Free IVDR GSPR Checklist [Template] Trinzo
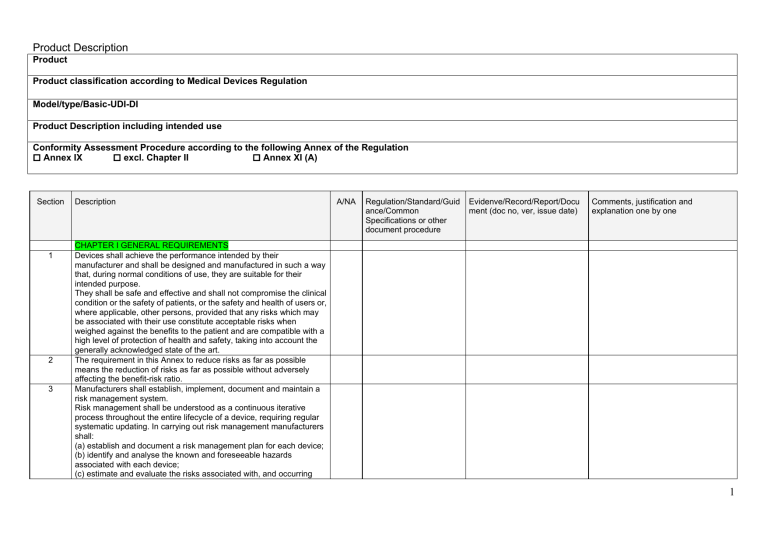
GSPR checklist
MDR General Safety and Performance (GSPR) checklist
+GSPR+Checklist+-+SCREENSHOT+1+WATERMARK.png)
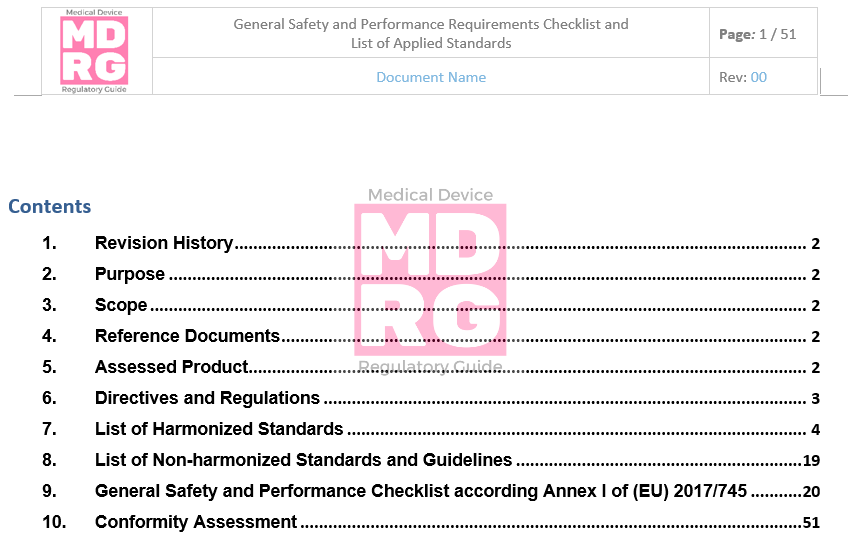
General Safety and Performance Requirements (GSPR) Checklist — Medical
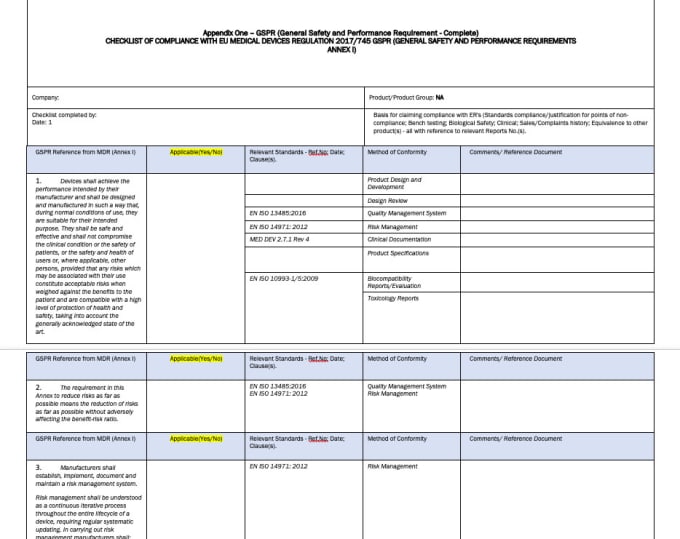
Mdr Gspr Checklist Template Portal Tutorials

Comparative table GSPR Essential Requirements (v3) Académie DM Experts
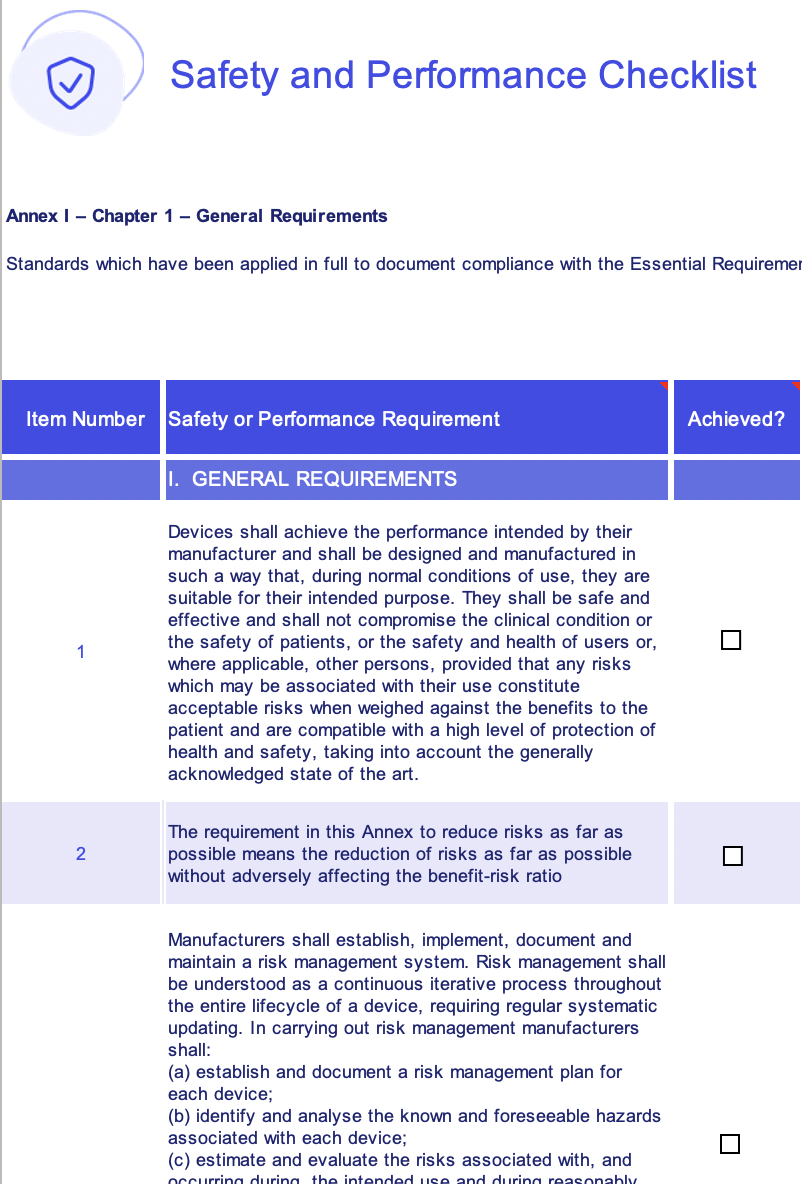
EU MDR general safety and performance requirements (GSPR) checklist
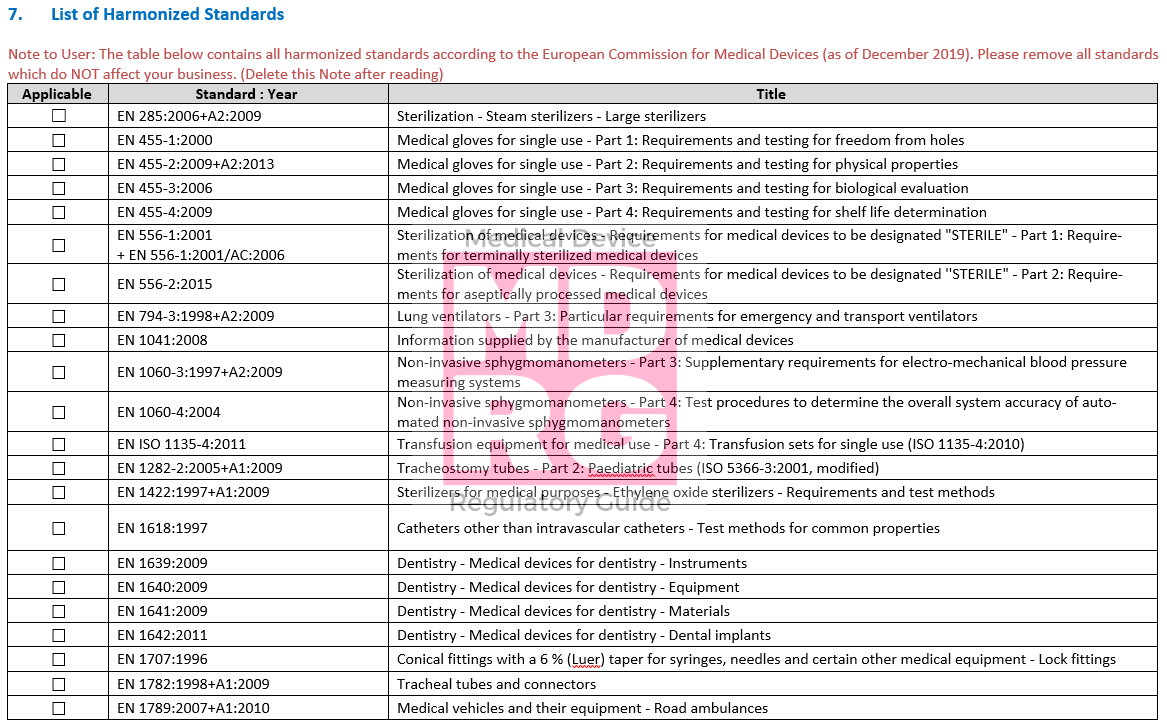+GSPR+Checklist+-+SCREENSHOT+2+WATERMARK.png)
General Safety and Performance Requirements (GSPR) Checklist — Medical
Web In The Gspr Checklist Clearly Mention The Below Points:
Web Mdrg Has Created A General Safety & Performance Requirements Checklist That Contains A Full Table Of The Requirements, Along With A List Of Applicable Standards.
Page 1 Of 10 # Requirement Standards Applied Design Documentation Qualification
This Excel Spreadsheet Is Designed To Support Manufacturers Making The Transition From Mdd To Mdr / Ivdd To Ivdr.
Related Post: