Fda Estar Template
Fda Estar Template - Web your estar submission questions answered. Find out how to use the estar template,. The food and drug administration (fda or agency) is announcing the availability of the draft guidance entitled “electronic submission. Web currently, the fda estar template can only be used to prepare 510 (k) and de novo submissions, but other types of submissions are in development, including. Web learn how to navigate the latest fda estar templates for medical devices, including pma submissions. Web we have also been telling our subscribers to anticipate a significant revision to the fda estar template when this happens. As the estar is a fillable pdf form, it contains questions for the user. Web cber's voluntary estar pilot program is intended to help applicants gain experience with the review of and internal processes for 510(k) submissions using the. 30 day free trialtrusted by millionsedit on any devicemoney back guarantee Starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using. As the estar is a fillable pdf form, it contains questions for the user. Find out how to use the estar template,. The food and drug administration (fda or agency) is announcing the availability of the draft guidance entitled “electronic submission. Web the fda has just released a version 4 template for the nivd and ivd templates, with version 3. Food and drug administration (fda) is announcing that you may now send electronic copy (ecopy) or electronic submission template and resource. Web the fda has just released a version 4 template for the nivd and ivd templates, with version 3 estar to be retired on august 13, 2023. Web currently, the fda estar template can only be used to prepare. Find out how to use the estar template,. Web learn how to navigate the latest fda estar templates for medical devices, including pma submissions. Web the fda has just released a version 4 template for the nivd and ivd templates, with version 3 estar to be retired on august 13, 2023. Web the estar, or electronic submission template and resource,. Web learn how to navigate the latest fda estar templates for medical devices, including pma submissions. Starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using. The food and drug administration (fda or agency) is announcing the availability of the draft guidance entitled “electronic submission. Web cber's voluntary estar pilot program is intended. The release of the updated estar. The food and drug administration (fda or agency) is announcing the availability of the draft guidance entitled “electronic submission. Web the current estar templates can be found on fda’s website: Web fda’s estar is an interactive pdf template for medical device 510 (k), de novo, and pma submissions. Find out the actionable steps, updates,. Web cber's voluntary estar pilot program is intended to help applicants gain experience with the review of and internal processes for 510(k) submissions using the. Find out the actionable steps, updates, and considerations for your. The food and drug administration (fda or agency) is announcing the availability of the draft guidance entitled “electronic submission. Find out how to use the. The release of the updated estar. Web the fda has just released a version 4 template for the nivd and ivd templates, with version 3 estar to be retired on august 13, 2023. Web currently, the fda estar template can only be used to prepare 510 (k) and de novo submissions, but other types of submissions are in development, including.. Web your estar submission questions answered. Web the current estar templates can be found on fda’s website: Web learn about the fda estar program, a voluntary electronic submission process for 510 (k) and de novo medical device applications. Find out how to use the estar template,. Web the fda has just released a version 4 template for the nivd and. Web we have also been telling our subscribers to anticipate a significant revision to the fda estar template when this happens. Starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using. Web estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical device submission to the. Find out how to use the estar template,. Find out the actionable steps, updates, and considerations for your. Web currently, the fda estar template can only be used to prepare 510 (k) and de novo submissions, but other types of submissions are in development, including. Web the current estar templates can be found on fda’s website: Web learn about the. Find out how to use the estar template,. Web learn how to navigate the latest fda estar templates for medical devices, including pma submissions. Starting on october 1, 2023, all 510(k) submissions, unless exempted, will need to be submitted electronically using. Web estar is an interactive pdf form that guides applicants through the process of preparing a comprehensive medical device submission to the fda. The release of the updated estar. The food and drug administration (fda or agency) is announcing the availability of the draft guidance entitled “electronic submission. Web we have also been telling our subscribers to anticipate a significant revision to the fda estar template when this happens. Web currently, the fda estar template can only be used to prepare 510 (k) and de novo submissions, but other types of submissions are in development, including. Web cber's voluntary estar pilot program is intended to help applicants gain experience with the review of and internal processes for 510(k) submissions using the. Web the estar, or electronic submission template and resource, is a free interactive pdf form designed to guide applicants through the process of preparing a. Web the current estar templates can be found on fda’s website: Web learn about the fda estar program, a voluntary electronic submission process for 510 (k) and de novo medical device applications. Web fda’s estar is an interactive pdf template for medical device 510 (k), de novo, and pma submissions. As the estar is a fillable pdf form, it contains questions for the user. Food and drug administration (fda) is announcing that you may now send electronic copy (ecopy) or electronic submission template and resource.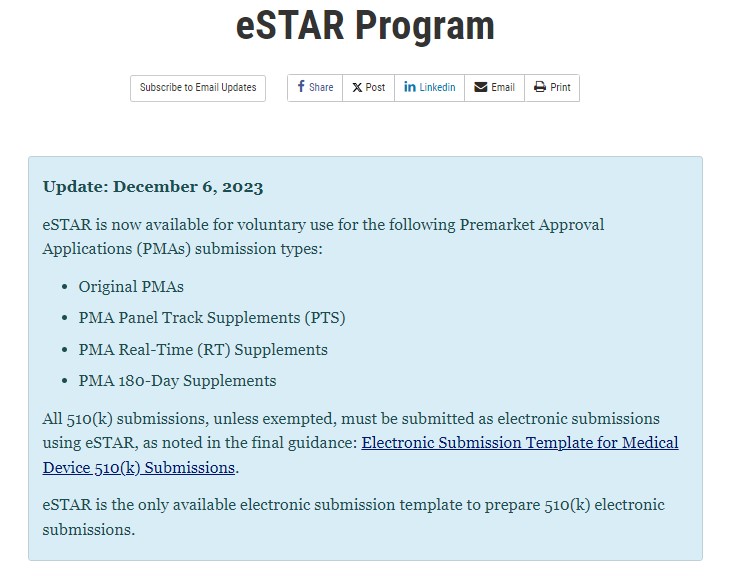
FDA updates their eSTAR templates to submit certain PMA applications

FDA Reports 58 Free Templates in PDF, Word, Excel Download

Using the new eSTAR templates for a 510(k) submission and the FDA eSTAR

Your Guide to the FDA eSTAR program Essenvia

What is the FDA eSTAR program?

Medhealth Review FDA eSTAR Application Submission Tips

About U.S FDA eSTAR eStarHelper

Health Canada announces a pilot with the FDA eSTAR template Medical

Form FDA 1572 PDF Food and Drug Administration Fill Out and Sign
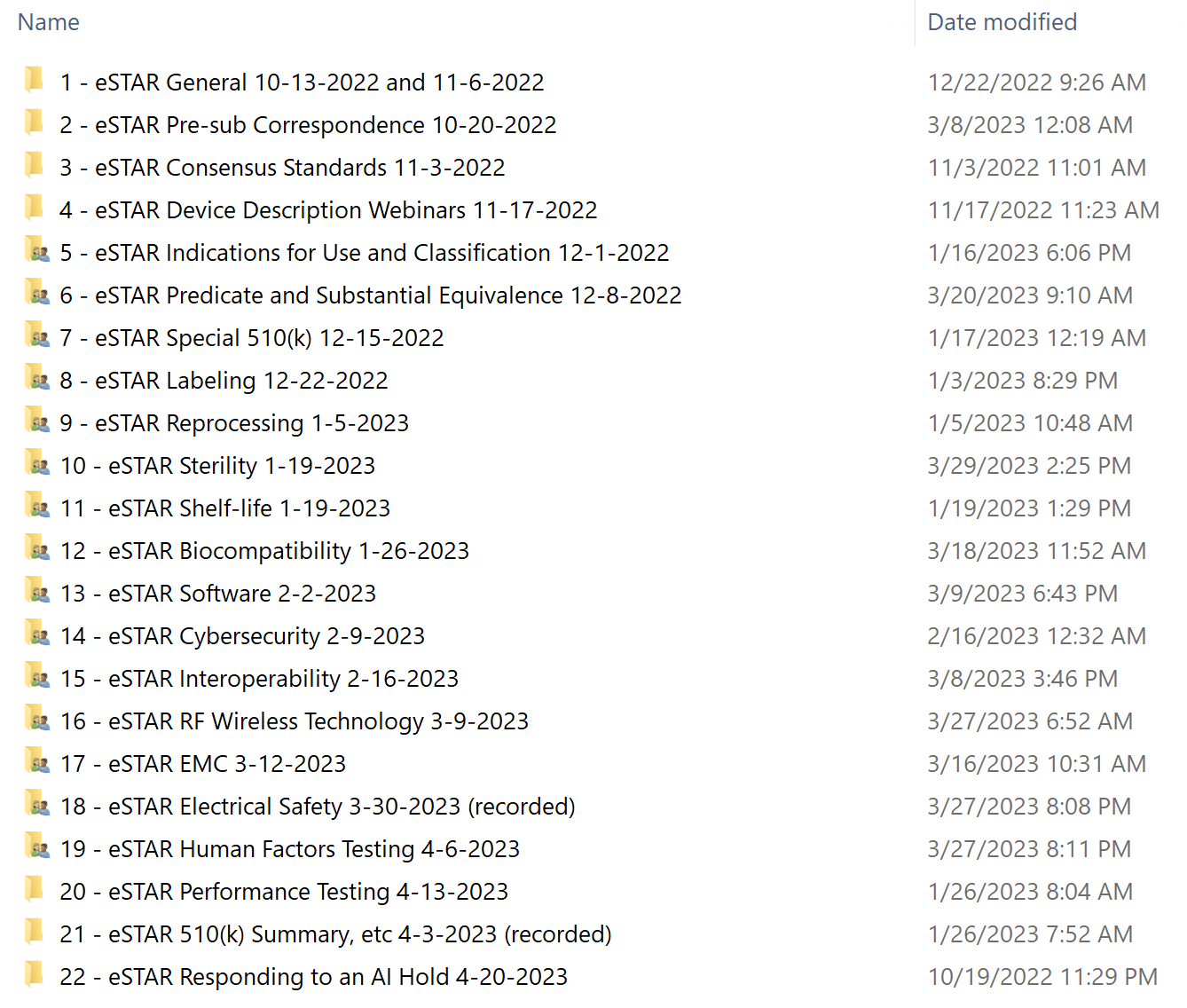
510k Course for FDA eSTAR 58+ Videos, Templates, and eBook
Web The Fda Has Just Released A Version 4 Template For The Nivd And Ivd Templates, With Version 3 Estar To Be Retired On August 13, 2023.
Web Your Estar Submission Questions Answered.
30 Day Free Trialtrusted By Millionsedit On Any Devicemoney Back Guarantee
Find Out The Actionable Steps, Updates, And Considerations For Your.
Related Post: