Electronegativity Pattern Periodic Table
Electronegativity Pattern Periodic Table - Web electronegativity is defined as an atom’s ability to attract electrons towards it in a chemical bond. Web understanding the periodic table's layout is key to remembering electronegativity trends. Electronegativity values generally increase from left to right across the periodic table. Web electronegativity varies across the periodic table in a well understood pattern, meaning that it is possible to use the periodic table of elements to predict the behaviour of different elements in combination (substances made of different elements in combination are known as compounds). Electronegativity , ionization energy , electron affinity , atomic radius , melting point, and metallic character. Web electronegativity is a measure of an atom's ability to attract shared electrons to itself. Electronegativity is the measure of an element’s ability to attract a bonding pair of electrons towards itself. Because cl lies above and to the right of se, we can predict that χ cl > χ se. This table is a list of electronegativity values of the elements. Electronegativity follows a trend (periodicity) on the periodic table. In general, electronegativity increases across a period because the number of protons increases while the number of shells stays the same. Electronegativity , ionization energy , electron affinity , atomic radius , melting point, and metallic character. Electronegativity is the measure of an element’s ability to attract a bonding pair of electrons towards itself. Web understanding the periodic table's layout. Web electronegativity trend in periodic table. Web values for electronegativity run from 0 to 4. As you move from left. It is caused by the attractive electrostatic force between the positively charged nucleus and the negatively charged electrons. Web understanding the periodic table's layout is key to remembering electronegativity trends. Electronegativities generally decrease from the top to bottom of a group. This color periodic table indicates each element's symbol, atomic number, and electronegativity. Web electronegativity is a measure of an atom's ability to attract shared electrons to itself. Cesium has an electronegativity value of 0.79. The trend is shown in the graphic (which is also available as a pdf for. Web understanding the periodic table's layout is key to remembering electronegativity trends. Web patterns of electronegativity in the periodic table. Electronegativity follows a trend (periodicity) on the periodic table. This means there is a greater charge which attracts on the shared electrons. Web values for electronegativity run from 0 to 4. Electronegativity increases moving left to right across a period, from the alkali metals to the halogens. This periodic table with electronegativity indicated can be downloaded in pdf format here. The distance of the electrons from the nucleus remains relatively constant in a periodic table row, but not in a periodic table column. Electronegativity values generally increase from left to right. Web a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). As we move across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electronegativity increases. This table is a list of electronegativity values of the elements. As you move from left. The trend is. Web updated on january 13, 2020. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. Web electronegativity trend in periodic table. This table is a list of electronegativity values of the elements. Electronegativity values generally increase from left to right across the periodic table. Web electronegativity is a measure of an atom's ability to attract shared electrons to itself. Web values for electronegativity run from 0 to 4. Across a period from left to right the electronegativity of atoms increases. The trend is shown in the graphic (which is also available as a pdf for printing). The distance of the electrons from the nucleus. This table is a list of electronegativity values of the elements. On the periodic table, electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. Since electro positivity is the exact opposite of electronegativity, cesium can be said to be the most electropositive element. Because cl lies above and. Ionization, radius, hardness, modulus, density, conductivity, heat, abundance, discovered. Web electronegativity is a measure of an atom's ability to attract shared electrons to itself. It was first described by linus pauling. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. A pdf of this periodic table. Web patterns of electronegativity in the periodic table. Pauling defined electronegativity as “the power of an atom in a molecule to attract electrons to itself.” Electronegativity , ionization energy , electron affinity , atomic radius , melting point, and metallic character. As you move from left. The force between two charges is given by coulomb’s law. Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound. The trend is shown in the graphic (which is also available as a pdf for printing). Electronegativity increases from left to right across a period due to the increasing positive charge in. In general, electronegativity increases across a period because the number of protons increases while the number of shells stays the same. Different elements have different electronegativities based on a number of factors such as size and number of protons, neutrons, and electrons. This color periodic table indicates each element's symbol, atomic number, and electronegativity. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Web a electronegativity increases from lower left to upper right in the periodic table (figure 2.12.2). This periodic table with electronegativity indicated can be downloaded in pdf format here. A pdf of this periodic table is available for printing. Web this entry was posted on may 7, 2014 by todd helmenstine (updated on may 24, 2021) this printable electronegativity periodic table shows the trends in electronegativity and values for each element.
Electronegativity Definition, Value Chart, and Trend in Periodic Table
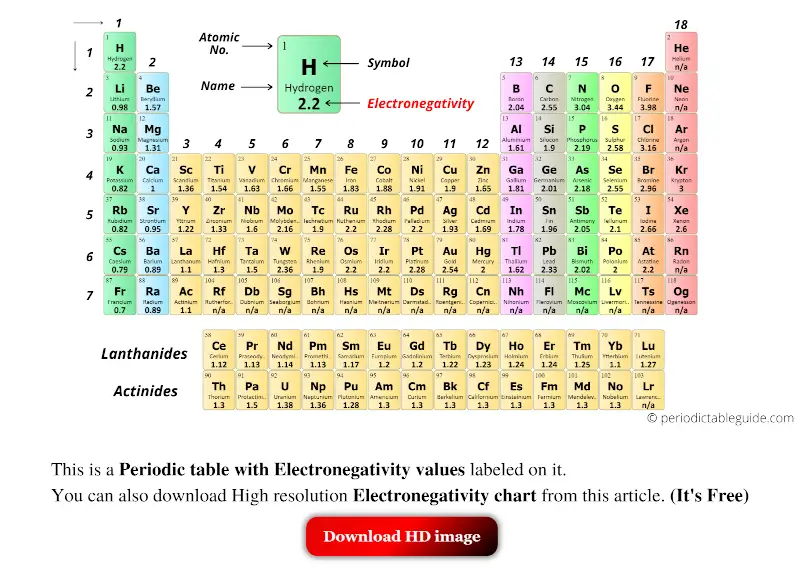
Electronegativity periodic table aftyred
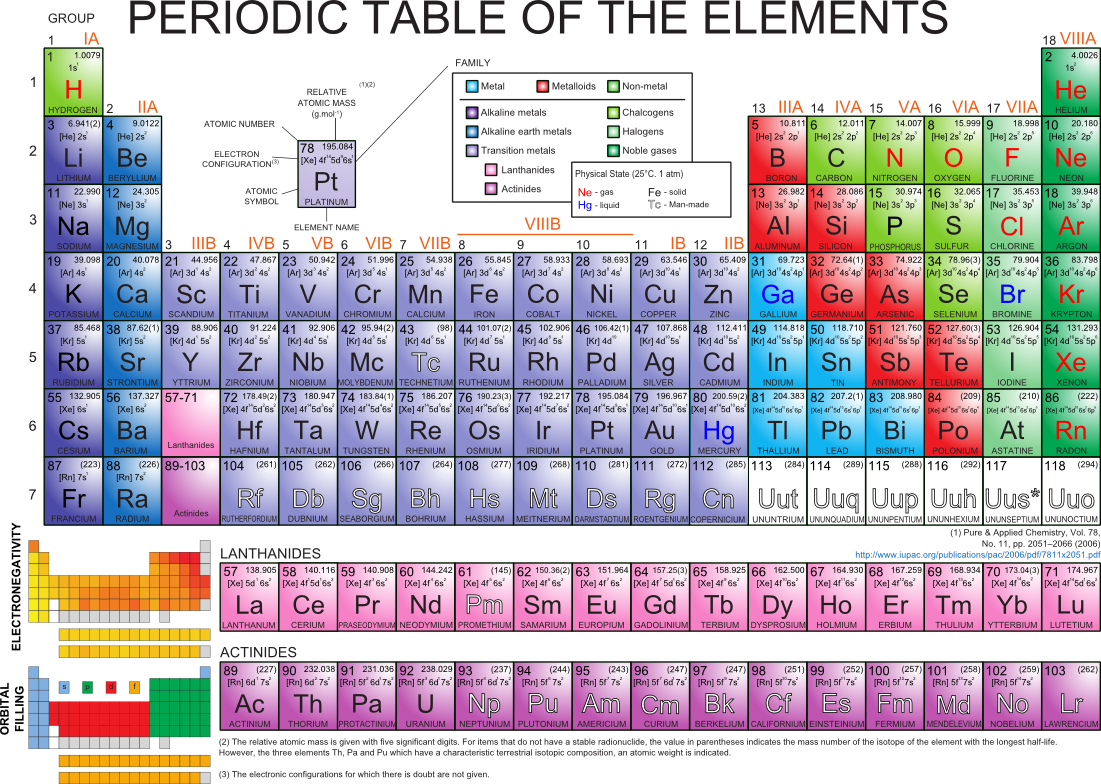
Periodic Table Of Elements Pdf With Electronegativity Elcho Table
Periodic Trends in Electronegativity CK12 Foundation

List of Electronegativity Values of the Elements
Periodic Table of Electronegativities

Electronegativity Definition and Trend
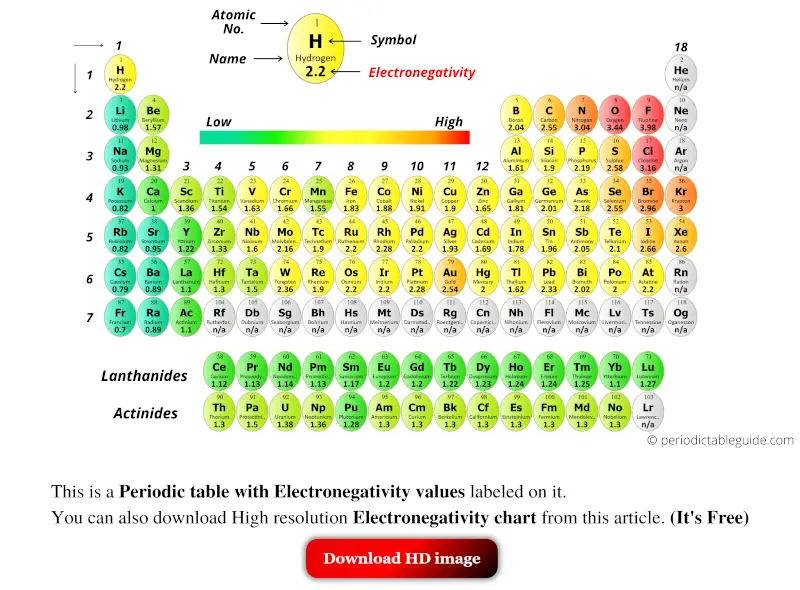
Periodic table with Electronegativity Values (Labeled Image)
/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png)
Printable Periodic Table of the Elements Electronegativity
Periodic Trends in Electronegativity CK12 Foundation
Web Electronegativity Is A Measure Of An Atom's Ability To Attract Shared Electrons To Itself.
Pdf Format Requires Adobe Acrobat Reader, Which Can Be Downloaded For.
Web Electronegativity Is Defined As An Atom’s Ability To Attract Electrons Towards It In A Chemical Bond.
The Distance Of The Electrons From The Nucleus Remains Relatively Constant In A Periodic Table Row, But Not In A Periodic Table Column.
Related Post:

.PNG)
