Drawing Solid Liquid Gas
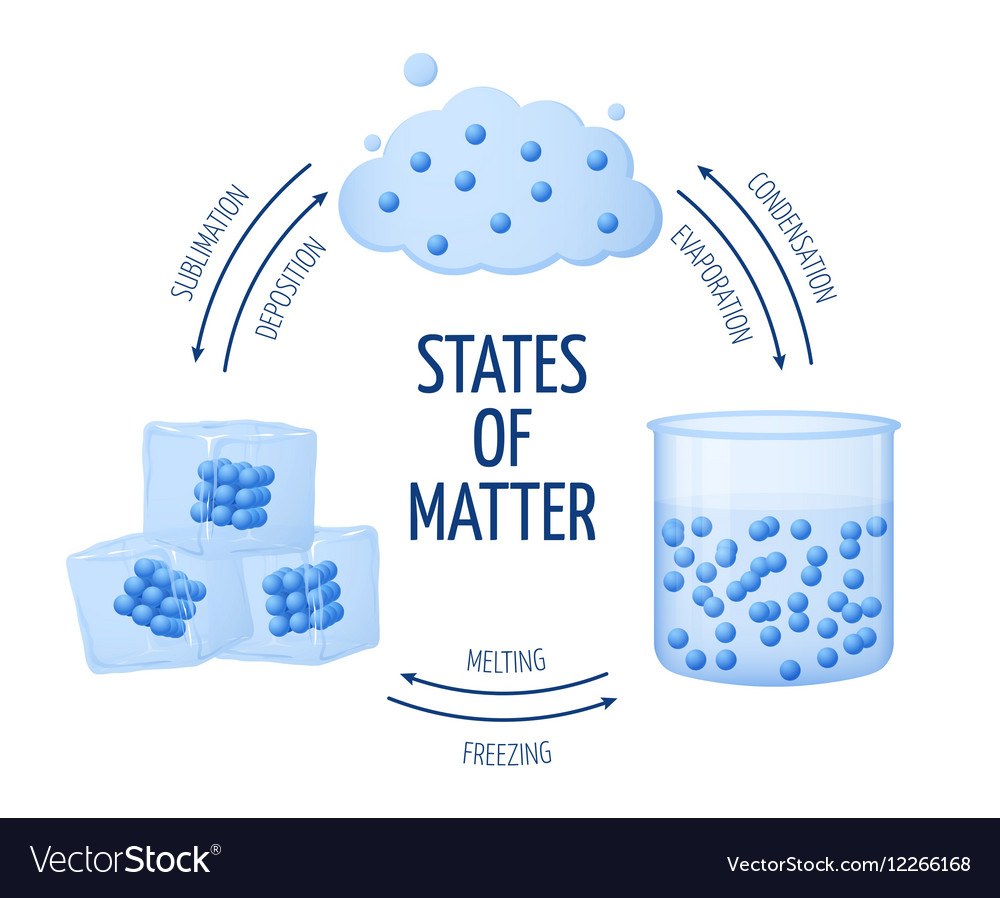
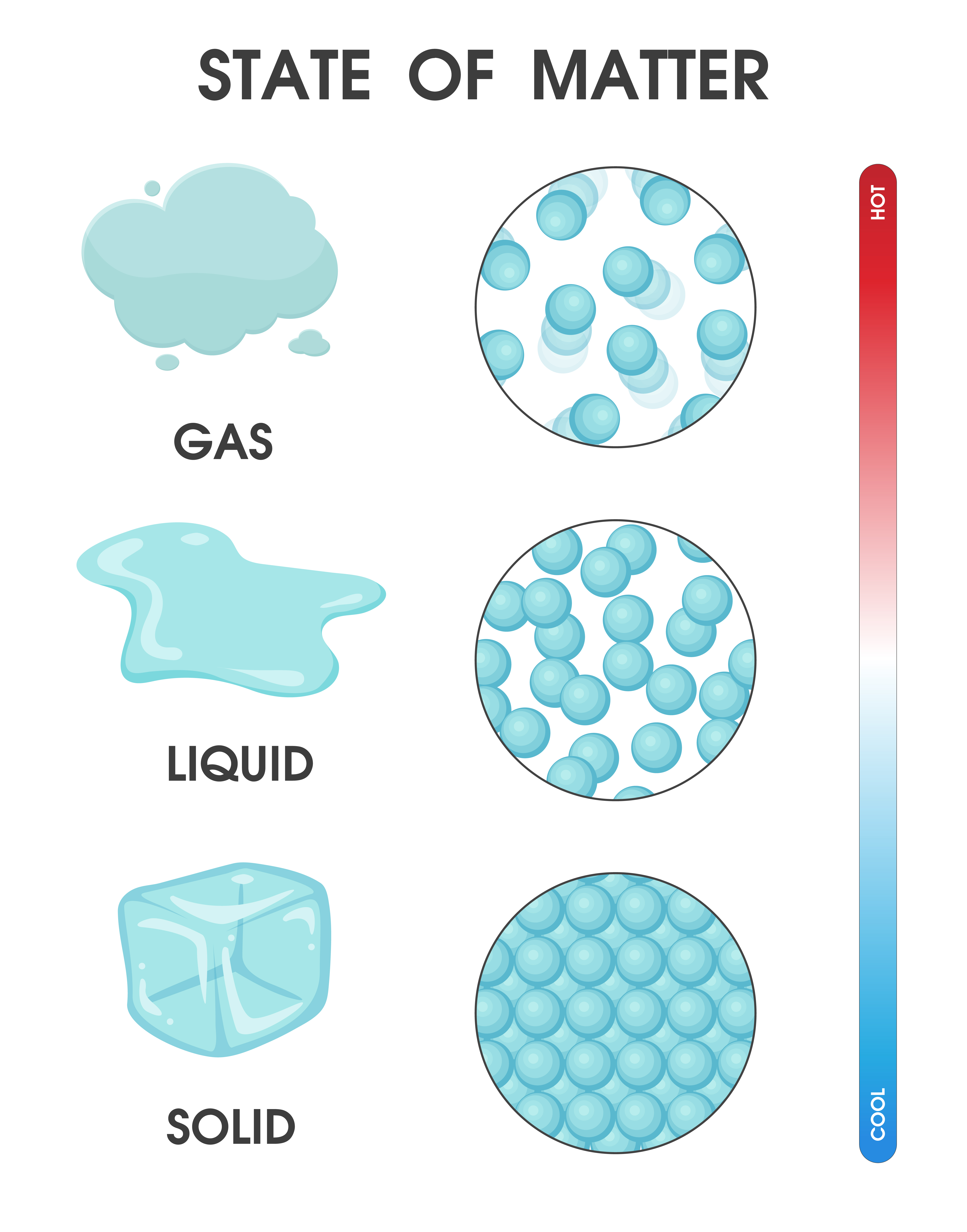
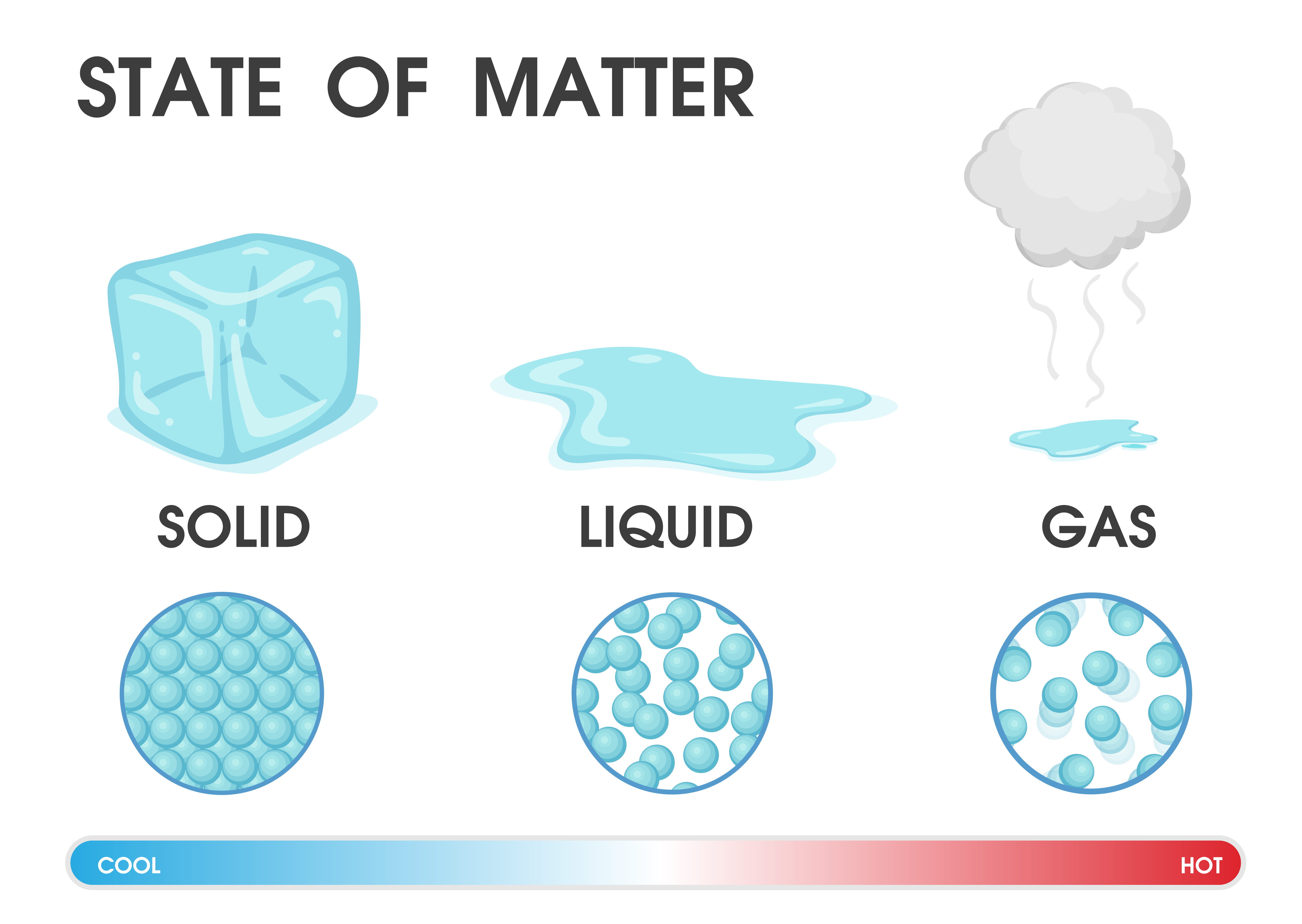
Drawing Solid Liquid Gas - The particle model explains the. A solid will retain its shape; Particles can be atoms, molecules or ions. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solid, liquids and gas are the three states of matter. The particles are not free to move around. The three states of matter can be represented by the particle model. Web solids, liquids, and gases are the three primary states of matter. The water molecules stay the same, but they behave differently as they change from one form to another. And why they adopt the shape (but not the volume) of their containers. The particles in a solid are either highly ordered (if the solid is crystalline) or have no regular arrangement (if the solid is amorphous). Web watch different types of molecules form a solid, liquid, or gas. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling. Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. Liquid matter is made of more loosely packed particles. Relate the interaction potential to the forces between molecules. The kinetic molecular theory of gases gives a reasonably accurate description of. The particles in a solid are either highly ordered (if the solid is crystalline) or have no regular arrangement (if the solid is amorphous). Matter that can change both shape and volume is called a gas. Web watch different types of molecules form a solid, liquid, or gas. Web in general covalent bonds determine: A gas will fill any container,. Topics covered in other articles. Almost everything is made of particles. Web this model explains the higher density, greater order, and lower compressibility of liquids versus gases; The particles are not free to move around. And why they adopt the shape (but not the volume) of their containers. A gas will fill any container, but if the container is not sealed, the gas will escape. The particles in a solid are either highly ordered (if the solid is crystalline) or have no regular arrangement (if the solid is amorphous). Solid matter is composed of tightly packed particles. Web in general covalent bonds determine: The water molecules stay the. Web solids, liquids, and gases are the three primary states of matter. Liquid matter is made of more loosely packed particles. Solid matter is composed of tightly packed particles. The particles are not free to move around. The particle model explains the. Matter that can change both shape and volume is called a gas. The particles in a solid are either highly ordered (if the solid is crystalline) or have no regular arrangement (if the solid is amorphous). Almost everything is made of particles. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter.. Gas can be compressed much more easily than. Add or remove heat and watch the phase change. Solid, liquids and gas are the three states of matter. The water molecules stay the same, but they behave differently as they change from one form to another. Web watch different types of molecules form a solid, liquid, or gas. Web watch different types of molecules form a solid, liquid, or gas. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Web matter that feels hard and maintains a fixed shape is called a solid; Web in this video, we'll learn how to represent solids, liquids, and gases using particulate models.. The following table summarizes properties of gases, liquids, and solids and identifies the microscopic behavior responsible for each property. Web in this video, we'll learn how to represent solids, liquids, and gases using particulate models. A gas will fill any container, but if the container is not sealed, the gas will escape. Relate the interaction potential to the forces between. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. Web kids draw examples of solids, liquids, and gases in this 1st grade physical science worksheet that focuses on the states of matter. Matter that can change both shape and volume is called a gas. The thermal expansion of liquids; Solid, liquids and gas are the three states of matter. Visit byju’s to learn more about it. The particles in a solid are either highly ordered (if the solid is crystalline) or have no regular arrangement (if the solid is amorphous). They vibrate and move freely at high speeds. Almost everything is made of particles. The three states of matter can be represented by the particle model. Web in this video, we'll learn how to represent solids, liquids, and gases using particulate models. Web liquids and solids are often referred to as condensed phases because the particles are very close together. Relate the interaction potential to the forces between molecules. Web in general covalent bonds determine: Understanding these states is crucial because they are fundamental concepts in chemistry and physics, and you might encounter phase changes in real life—from boiling water to freezing ice and condensation. Matter that feels wet and maintains its volume but not its shape is called a liquid.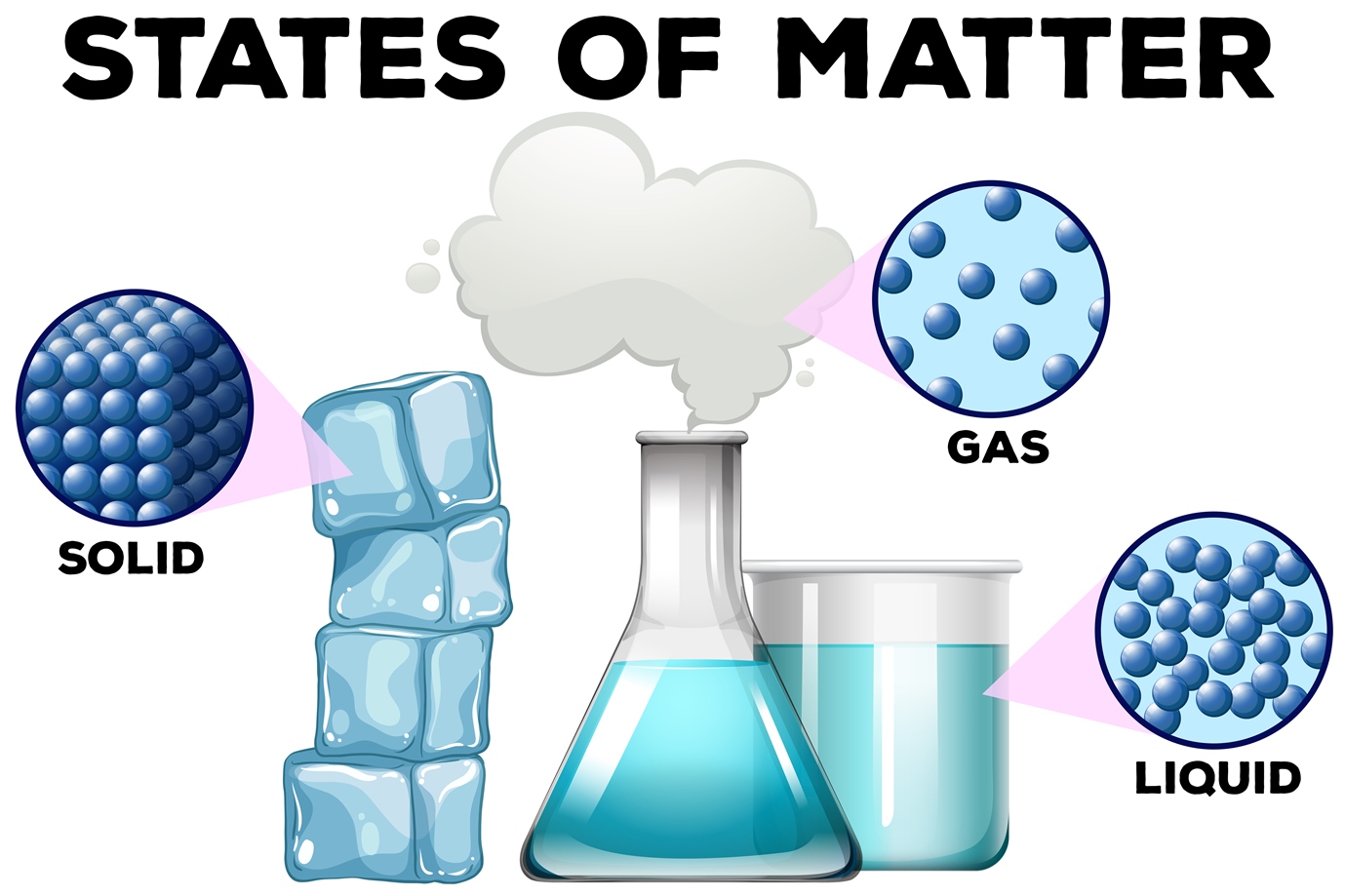
Solids, liquids and gases
Different states of matter solid liquid gas Vector Image
Changing the state of matter from solid, liquid and gas due to

state of matter drawing simple and easy solid liquid gases
Changing the state of matter from solid, liquid and gas due to
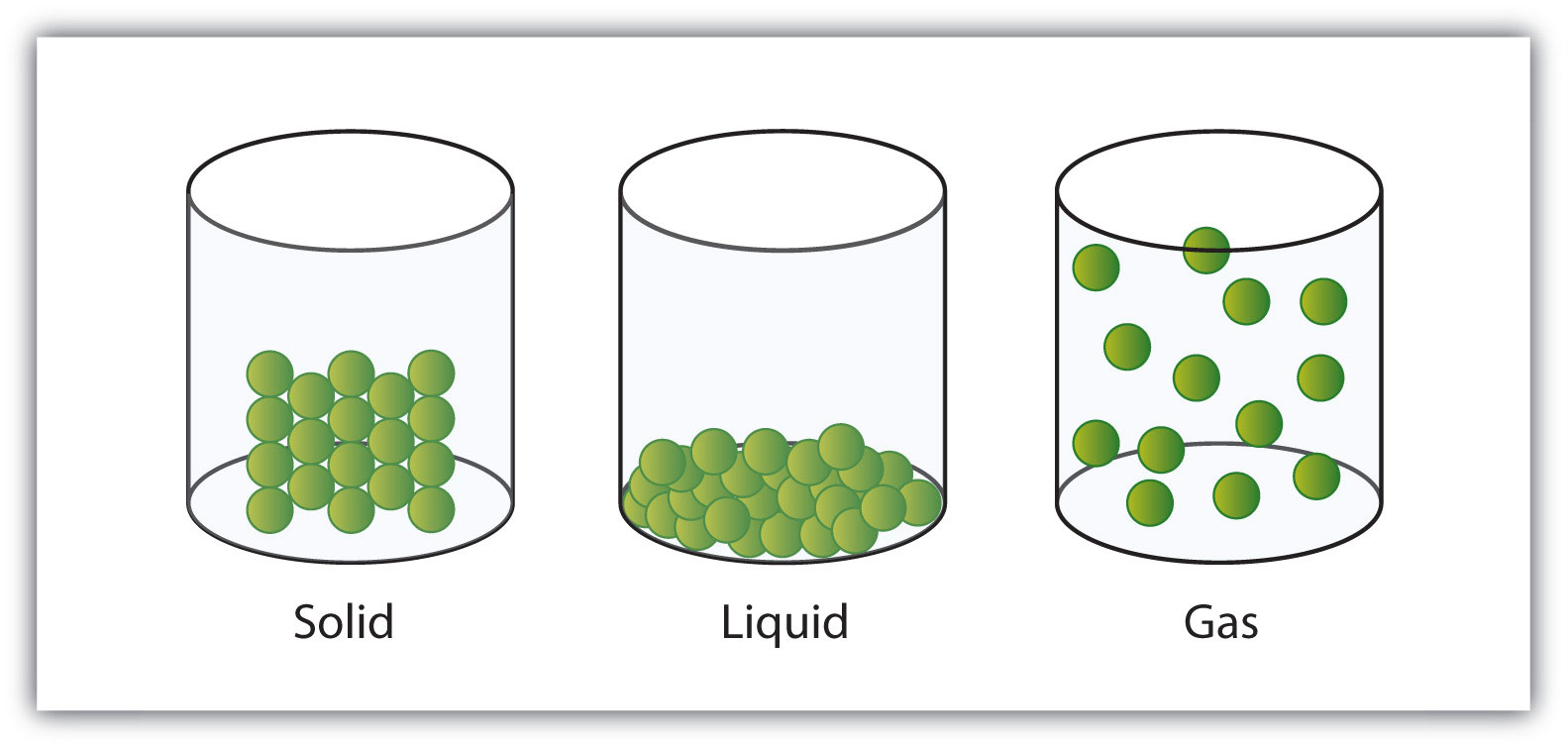
8.2 Solids and Liquids The Basics of General, Organic, and Biological
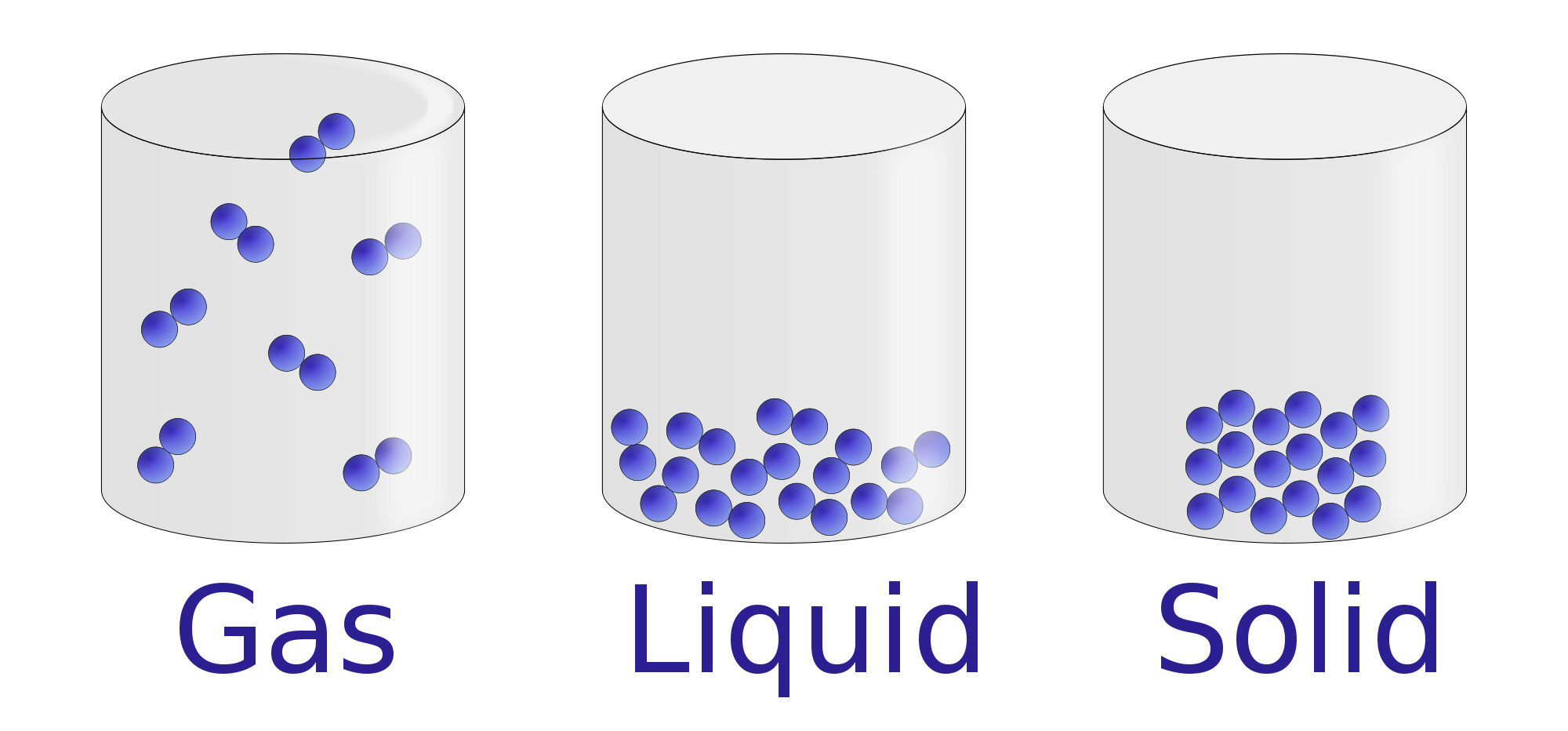
Properties of Liquids Chemistry Visionlearning
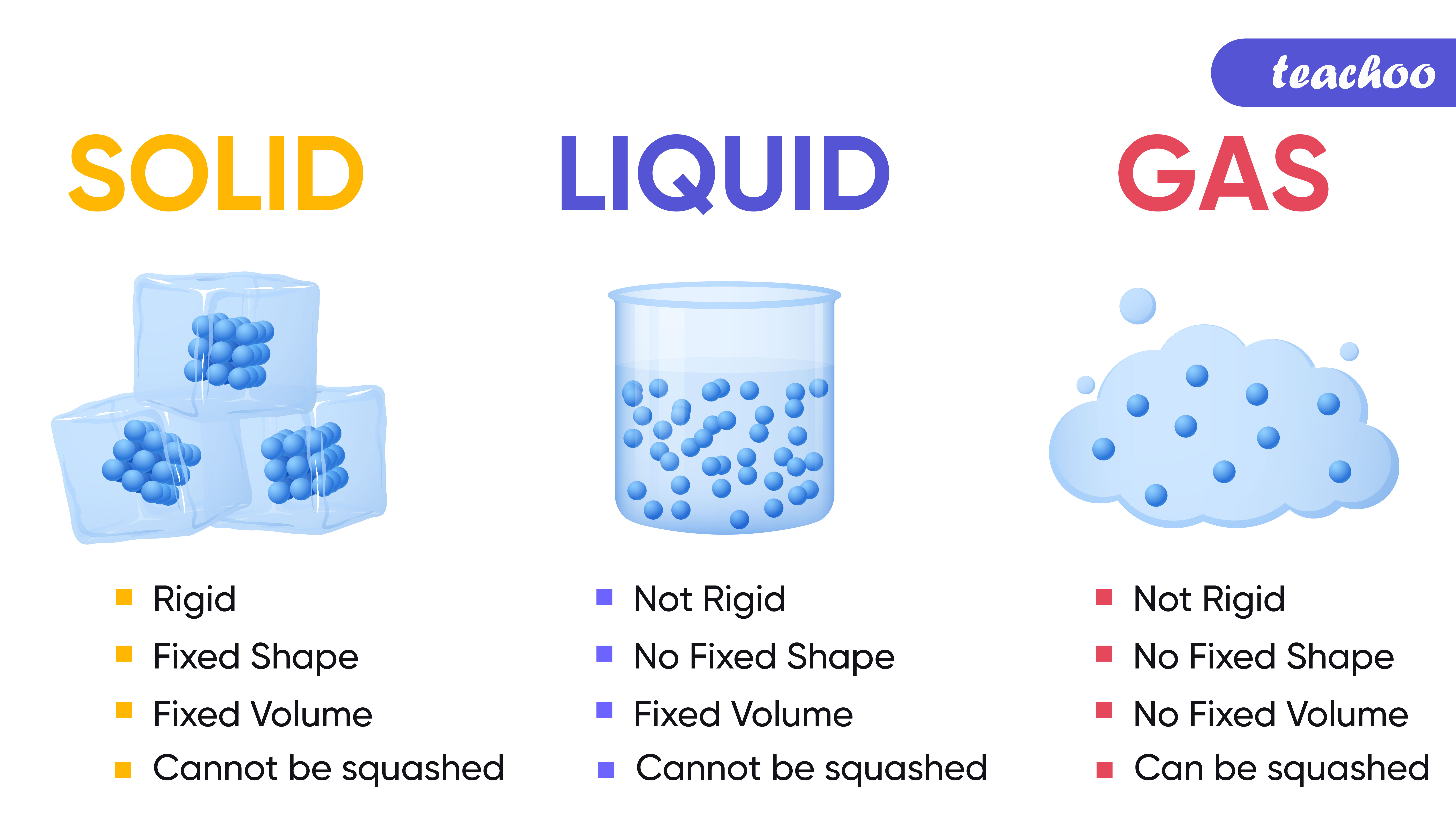
Properties of Solids, Liquids, Gases Compared Teachoo Science

Draw a sketch to show the arrangement of particles in solid, liquid and
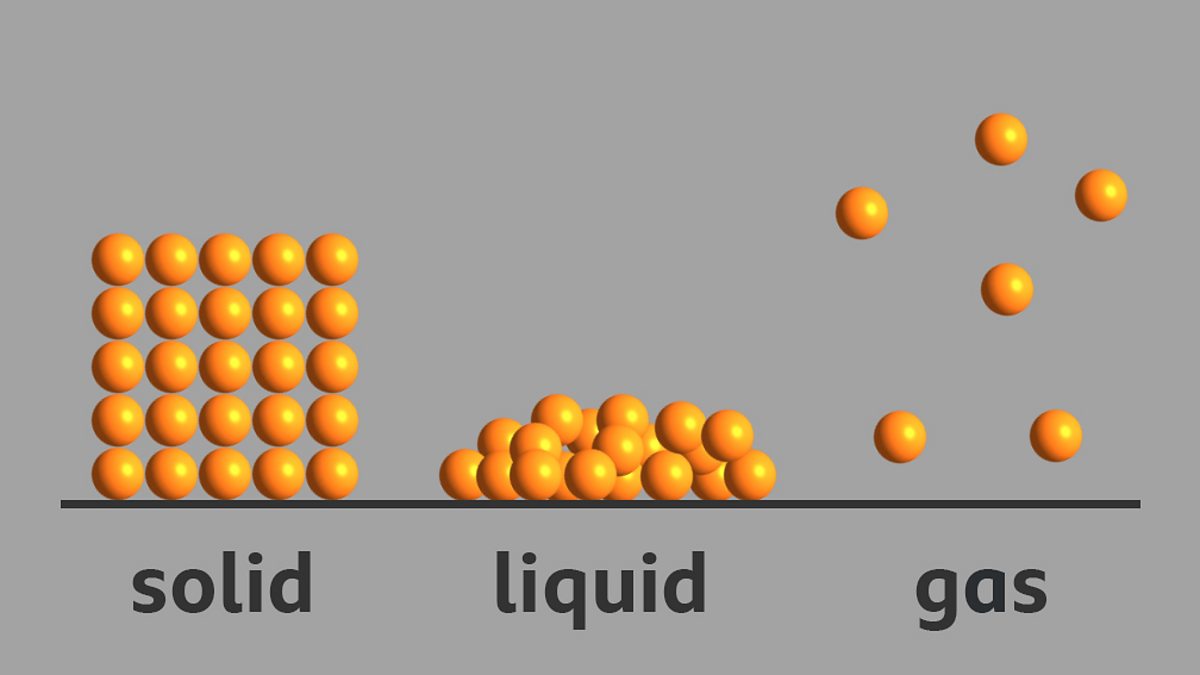
What is the arrangement of particles in a solid, liquid and gas? BBC
Liquid Matter Is Made Of More Loosely Packed Particles.
And Why They Adopt The Shape (But Not The Volume) Of Their Containers.
Particles Can Be Atoms, Molecules Or Ions.
Web This Model Explains The Higher Density, Greater Order, And Lower Compressibility Of Liquids Versus Gases;
Related Post: