Drawing Of Covalent Bond
Drawing Of Covalent Bond - Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. Example \(\pageindex{1}\) formula writing for covalent compounds. Web we refer to this as a pure covalent bond. Web draw lewis structures depicting the bonding in simple molecules. Determine the total number of valence electrons in the molecule or ion. Several socratic answers give the procedure. The following procedure can be used to draw lewis structure for simple molecules. Web how to draw a lewis structure. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web representing a covalent bond using lewis structures ; Examples for drawing lewis structures for covalent bonds. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. The following procedure can be used to draw lewis structure for simple molecules. Lewis structures are representations of molecules that include not only what atoms are present in the. Web representing a covalent bond using lewis structures ; How can i draw a lewis structure of a compound? The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Web a covalent bond is a chemical bond between two atoms where they share one or more pairs. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. However, some atoms will not give up or gain electrons easily. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the first shell.. Web a covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. A chemical bond resulting from two atoms sharing one or more pairs of electrons. Determine the total number of valence electrons in the molecule or ion. Draw lewis structures for covalent compounds. Examples for drawing lewis structures for covalent bonds. The only thing smaller than atoms is their subatomic particles; The two h atoms can share their electrons: Atoms form double or triple covalent bonds if they can attain a noble gas structure by doing so. Web draw lewis structures depicting the bonding in simple molecules. The following procedure can be used to draw lewis structure for simple molecules. Web representing a covalent bond using lewis structures ; Determine the total number of valence electrons in the molecule or ion. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. 4.7k views 7 years ago 1d: Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams,. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Web covalent bonds between different atoms. The following procedure can be used to draw lewis structure for simple molecules. In any molecule or. Practice with drawing lewis structures. Web as covalent bonds are formed by sharing of electrons between atoms, we draw overlapping circles to show the overlapping of the electron shells, and draw in pairs of dots and crosses to show the sharing of electrons. Covalent bonds usually form between nonmetals. Hydrogen is an exception to the octet rule. The only thing. In any molecule or ion with the general formula abn , the unique atom (a) is in the center and all of the b atoms are attached to a. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Web a covalent bond is a chemical bond. Exercise \(\pageindex{1}\) rules for drawing covalent lewis structures; 0:08 introduction 0:39 h2 1:25 hcl. However, some atoms will not give up or gain electrons easily. Web how to draw a lewis structure. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Hydrogen is an exception to the octet rule. Web we refer to this as a pure covalent bond. Hydrogen (h 2 ), chlorine (cl 2 ), oxygen (o 2 ), nitrogen (n 2 ), hydrogen chloride (hcl), water (h 2 o), ammonia (nh 3) and methane (ch 4) the correct dot and cross diagrams for these molecules are shown below: Exercise \(\pageindex{1}\) rules for drawing covalent lewis structures; Given descriptions, diagrams, scenarios, or chemical symbols, students will model covalent bonds using electron dot formula (lewis structures). Web define covalent bond. Usually, sharing electrons gives each atom a full valence shell and makes the resulting compound more stable than its constituent atoms are on their own. Drawing lewis structures for molecules with one central atom: The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Web a single line indicates a bond between two atoms (i.e., involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e., involving two electron pairs), and triple lines (≡) represent a triple bond, as found, for example, in. Web draw lewis structures for covalent compounds. Single, double, and triple covalent bonds depend on the number of pairs of electrons shared between two atoms. 0:08 introduction 0:39 h2 1:25 hcl. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Web draw lewis structures depicting the bonding in simple molecules. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.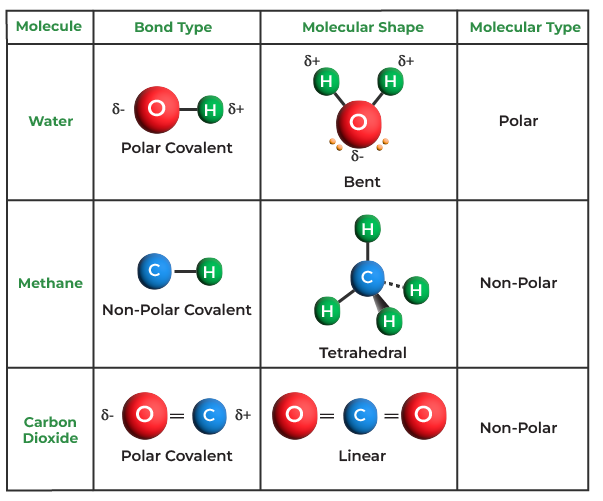
Covalent Bond Definition, Examples, Types, Properties, & FAQs

Covalent Bonding The Science and Maths Zone

Covalent bonding Dot and cross diagrams Graphite

Covalent Bonding The Science and Maths Zone

Covalent bonding tecscience

How To Draw Covalent Bonds

Introducing Covalent Bonding Montessori Muddle

How is a covalent bond formed

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Covalent bond (covalency) and its type Overall Science
Atoms Form Double Or Triple Covalent Bonds If They Can Attain A Noble Gas Structure By Doing So.
Web And Introductory Video For My General Chemistry Class (Goes Along With Notes Packet #9) Showing Lewis Dot Structures For Simple Molecules Involving Single, D.
Rules For Writing Covalent Formulas;
Determine The Total Number Of Valence Electrons In The Molecule Or Ion.
Related Post: