Drawing Ionic Compounds
Drawing Ionic Compounds - Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. What would be the charge on chlorine? 298k views 3 years ago new ap & general chemistry video playlist. Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Web draw lewis structures for ionic compounds. Draw the lewis structure the ionic compound of barium fluoride the fluorine is full, but the barium. Ionic structures (worksheet) page id. Web draw lewis diagrams for each of these elements. When discussing the octet rule, we do not consider d or f. Web the steps to draw the lewis structure of an ionic compound are: Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. Sat sep 07, 2019 7:16 am. Activities 1 and 2 introduce ionic bonding dot and cross diagrams in a format that allows learners to. Postby amir bayat » sun nov 03, 2019 7:55 pm. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Compounds can be classified as ionic or covalent. Only give reasonable results for covalent compounds and polyatomic ions. This chemistry video explains how to draw the lewis structures of. Web draw the lewis structure the ionic compound of barium fluoride 4: Determine the charge of each ion based on the element's position in the periodic table. To begin, you must know two essential rules for drawing. Only give reasonable results for covalent compounds and polyatomic ions of the. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. Web to draw ionic compounds, draw the lewis structures of each ion individually, and label the charge of each and put them in brackets. This chemistry video explains how to draw the lewis structures of. Web it's just for ionic compounds electrons aren't. Web the organic chemistry tutor. Remember that lewis dot structures. Web draw lewis structures for ionic compounds. Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Many of the ions that form have eight electrons in their valence shell. Web to draw ionic compounds, draw the lewis structures of each ion individually, and label the charge of each and put them in brackets. Ethanethiol, c a 2 h a 6 s , is a clear liquid with a strong odor. Ionic structures (worksheet) page id. When drawing lewis dot structures for ionic compounds you need to follow a different. Sat sep 07, 2019 7:16 am. 298k views 3 years ago new ap & general chemistry video playlist. The astute reader may have noticed something: Magnesium oxide dot & cross diagram. Web draw lewis structures for ionic compounds. Identify the elements involved in the compound and determine their respective valence electrons. Web draw the lewis structure the ionic compound of barium fluoride 4: To begin, you must know two essential rules for drawing. Show how electrons are transferred in ionic bonding. Ionic structures (worksheet) page id. Web the organic chemistry tutor. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Ionic structures (worksheet) page id. What would be the charge on chlorine? Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not. In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons. 298k views 3 years ago new ap & general chemistry video playlist. Web shows how to draw lewis dot structures for ionic compounds. The astute reader may have noticed something: Many of the ions that form have eight electrons in their valence shell. Add more atoms if needed if the transfer from one atom to another doesn’t result in full outer shells, add more atoms ba f barium has 2 electron fluorine has 7 electrons example: The compound is often added to otherwise odorless fuels such as natural gas to help warn of gas leaks. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. To begin, you must know two essential rules for drawing. What would be the charge on chlorine? The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. During ionic bonding the atoms form ions by magnesium has two electrons in its outer gaining or losing electrons to obtain a full outer shell, oxygen has six. Web draw lewis diagrams for each of these elements. Instead ionic compounds stick together through electrostatic forces (different electrically charged ions) which we usually represent with brackets and the charge in the upper right corner. For exam purposes you need only show the outer electrons in dot & cross diagrams.you should be able to draw dot & cross diagrams for combinations of ions from groups 1,2,3,5,6 and 7. Web to draw ionic compounds, draw the lewis structures of each ion individually, and label the charge of each and put them in brackets.
What Is An Ionic Compound? Formula and Defination
/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
Examples of Ionic Bonds and Compounds
Diagram of an Ionic Compound Stock Illustration Illustration of
Examples of Ionic Bonds and Compounds

ionic bond Definition, Properties, Examples, & Facts Britannica

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk

savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures

What are Ionic Compounds and how they are formed?
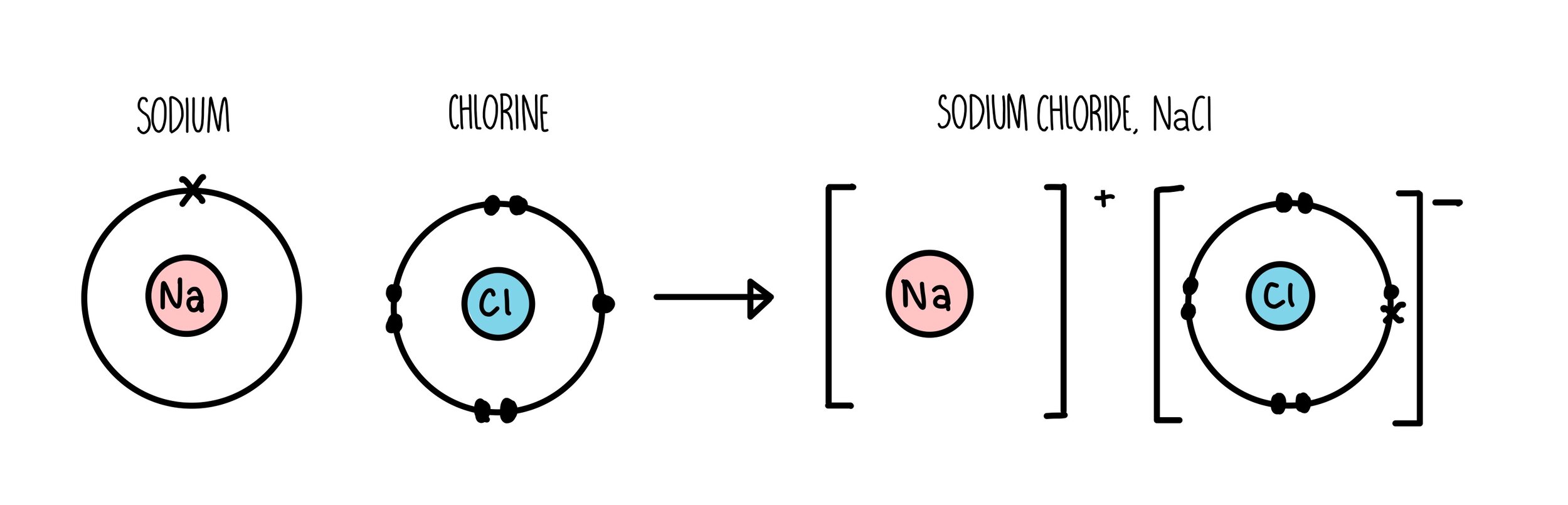
Ionic Bonding — the science hive
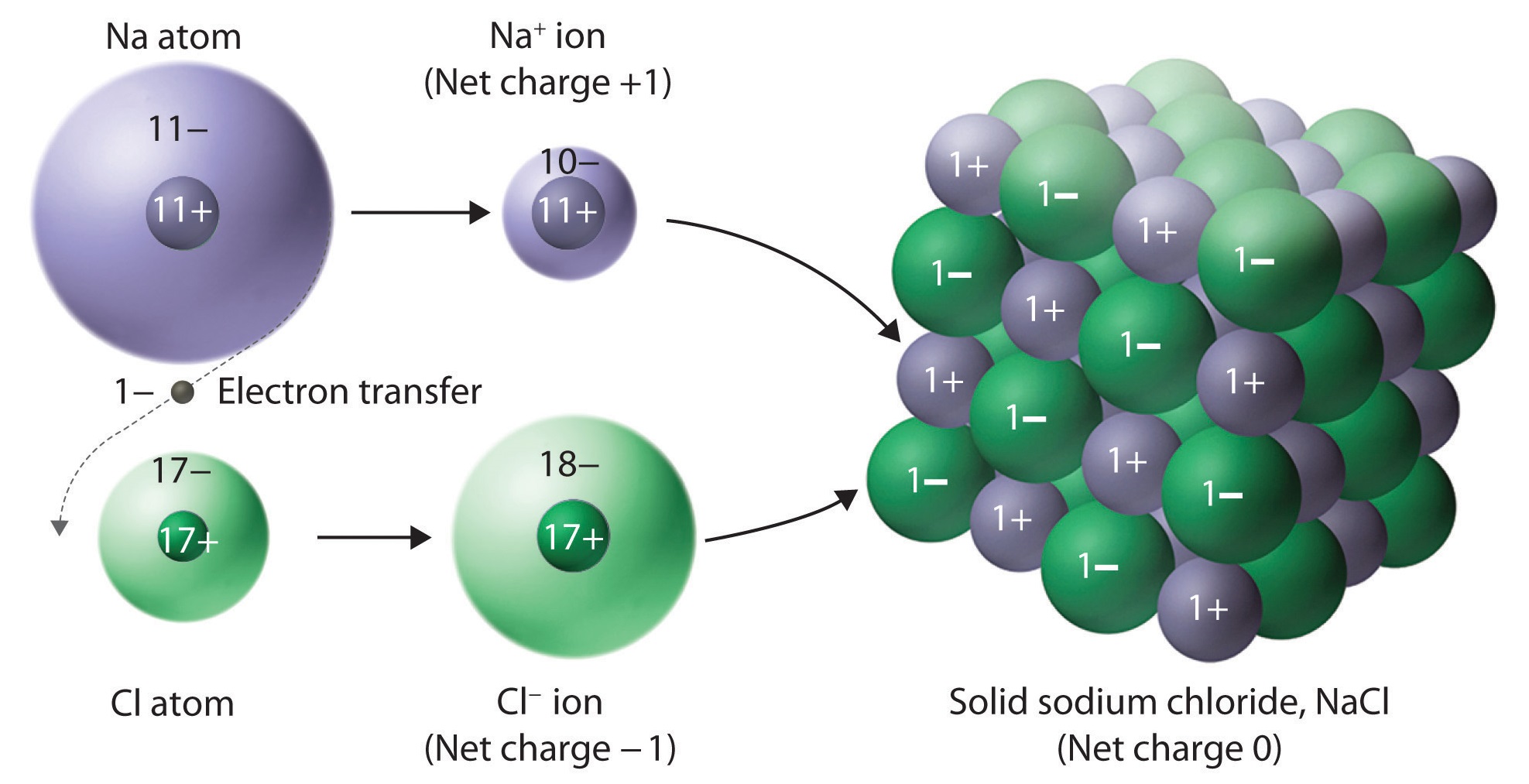
Ionic Solids Chemistry LibreTexts
Sat Sep 07, 2019 7:16 Am.
When Drawing Lewis Dot Structures For Ionic Compounds You Need To Follow A Different Set Of Rules Than With Lewis Structures For Covalent/Molecular Compounds.
Show How Electrons Are Transferred In Ionic Bonding.
Web The Steps To Draw The Lewis Structure Of An Ionic Compound Are:
Related Post: