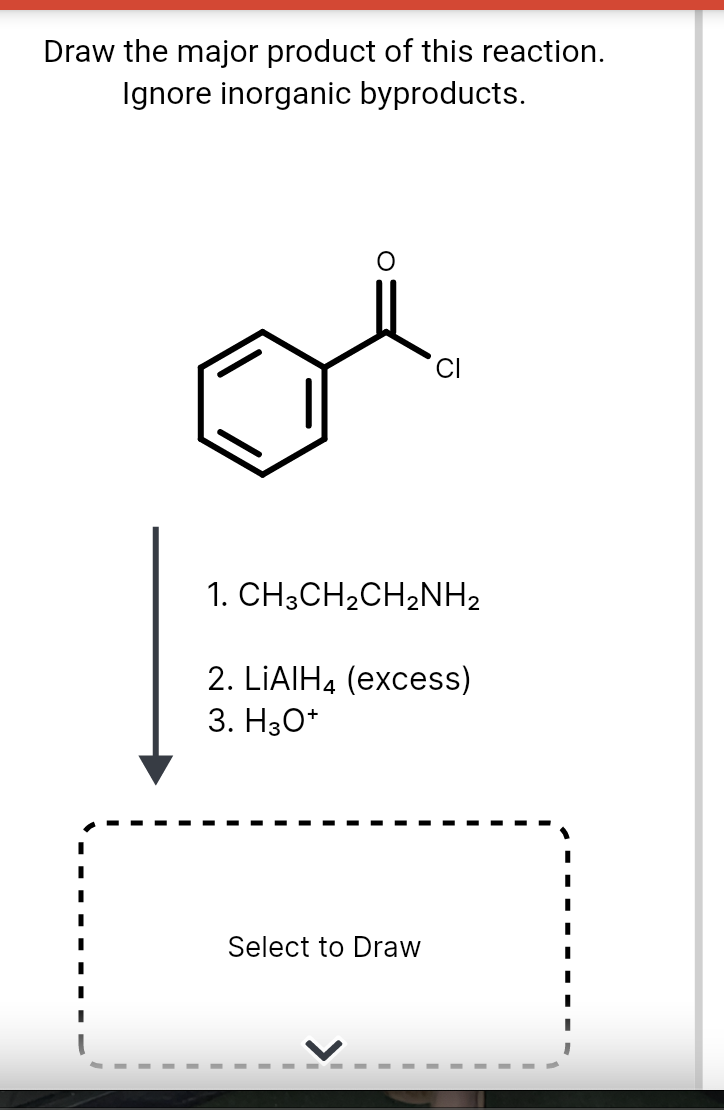
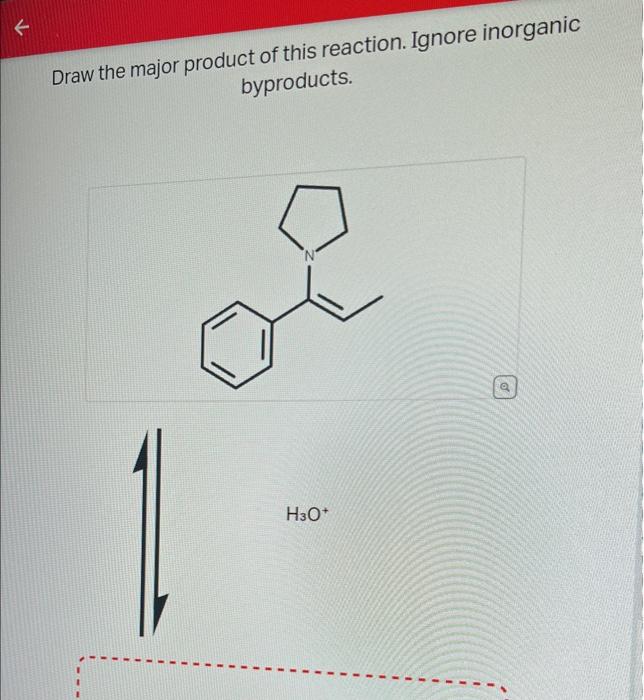
Draw The Product Of This Reaction Ignore Inorganic Byproducts
Draw The Product Of This Reaction Ignore Inorganic Byproducts - Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state. This problem has been solved! Draw the starting structure that would lead to the major product shown under the provided conditions. Draw the major product of this reaction. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. So, the product of this reaction will be a molecule with two. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The balanced chemical equation for the reaction is: Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Web ignore any inorganic byproducts. The product of this reaction is nh3. Draw the major product of this reaction. So, the product of this reaction will be a molecule with two. Web hbr (1 equiv) select to draw draw the product of this reaction. Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The product of this reaction is nh3. The balanced chemical equation for the reaction is: Draw the major product of this reaction. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. Web hbr (1 equiv) select to draw draw the product of this reaction. The product of this reaction is nh3. Draw a terminal alkene that would lead to this as the major product under these conditions.. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The balanced chemical equation for the reaction is: O 2 \mathrm{o_2} o 2 Draw the starting structure that would lead to the major product shown under the provided conditions. The product of this reaction is nh3. Web ignore any inorganic byproducts. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final. Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. Draw the major product of this reaction. This problem has been solved! Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. So, the product of this reaction. This problem has been solved! Draw a terminal alkene that would lead to this as the major product under these conditions. Br2 (2 equiv) select to draw draw the starting structure that would produce this product under these conditions. So, the product of this reaction will be a molecule with two. Sodium goes from a 0 to +1 oxidation state. The product of this reaction is nh3. Draw the starting structure that would lead to the major product shown under the provided conditions. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The balanced chemical equation for the reaction is: This problem has been solved! Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Web hbr (1 equiv) select to draw draw the product of this reaction. So, the product of this reaction will be a molecule. Therefore, the product of this reaction is \textbf{nh3}. Finally, the addition of h3o+ will protonate the negatively charged carbon, resulting in the final product. Web hbr (1 equiv) select to draw draw the product of this reaction. The balanced chemical equation for the reaction is: O 2 \mathrm{o_2} o 2 Therefore, the product of this reaction is \textbf{nh3}. Draw the major product of this reaction. Web 2na(s) + cl 2(g) → 2nacl(s) this reaction can also be classified as a redox reaction due to the changes in oxidation states. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web ignore any inorganic byproducts. The product of this reaction is nh3. So, the product of this reaction will be a molecule with two. This problem has been solved! Draw a terminal alkene that would lead to this as the major product under these conditions. Sodium goes from a 0 to +1 oxidation state while chlorine goes from a 0 to a − 1 oxidation state. The balanced chemical equation for the reaction is: O 2 \mathrm{o_2} o 2Solved Draw the product of this reaction. Ignore
[Solved] . Draw the product of this reaction. Ignore
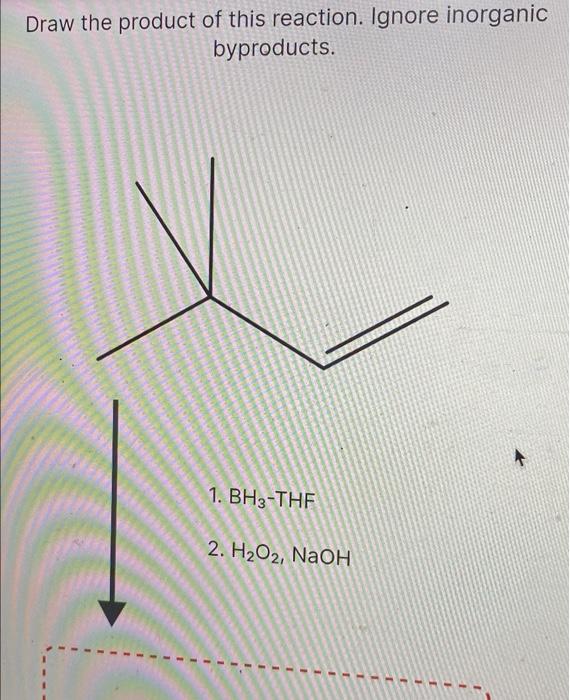
Solved Draw the product of this reaction. Ignore

draw the major product of this reaction. ignore byproducts
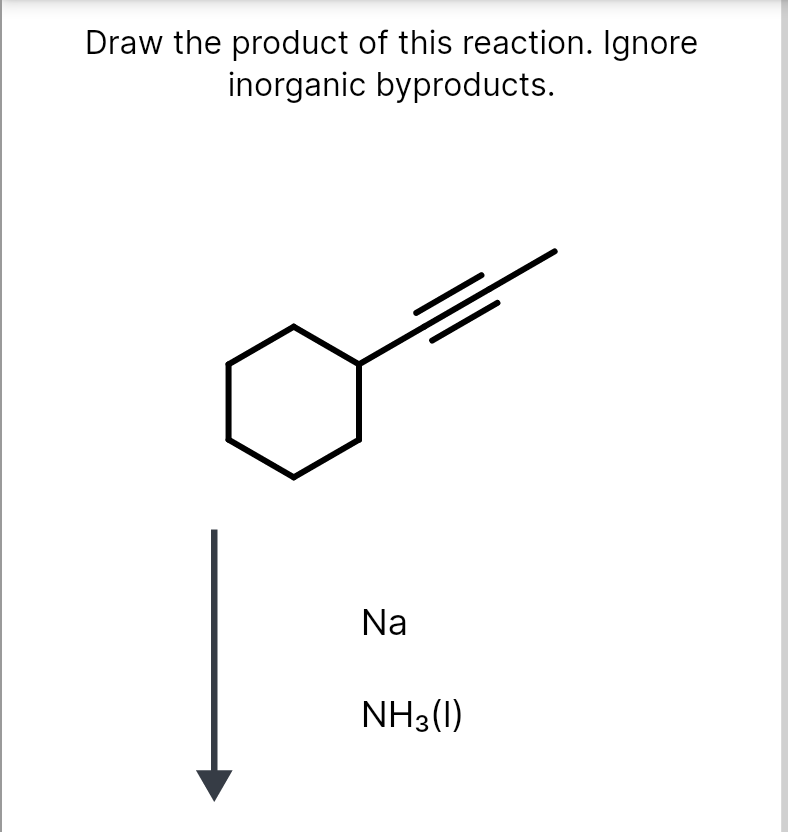
Solved Draw the major product of this reaction. Ignore
Solved Draw the major product of this reaction. Ignore
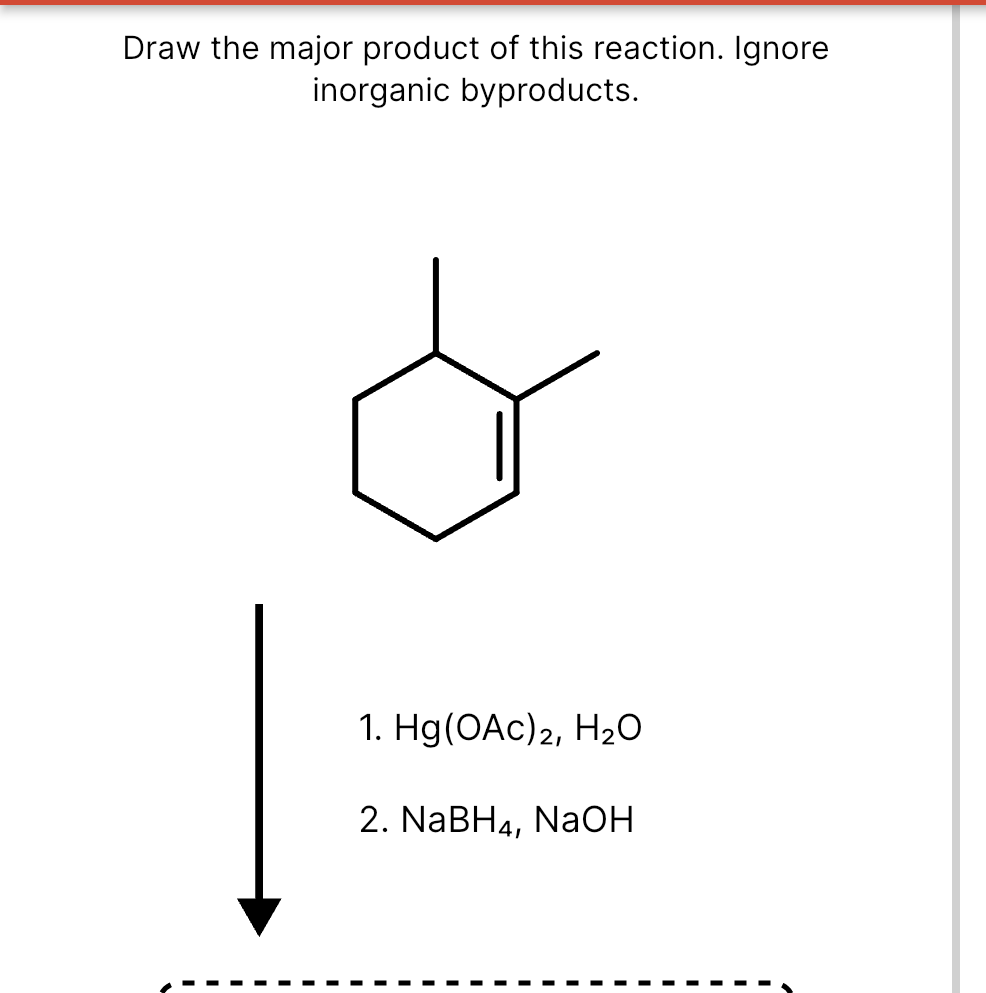
Solved Draw the major product of this reaction. Ignore
Solved Draw the major product of this reaction. Ignore
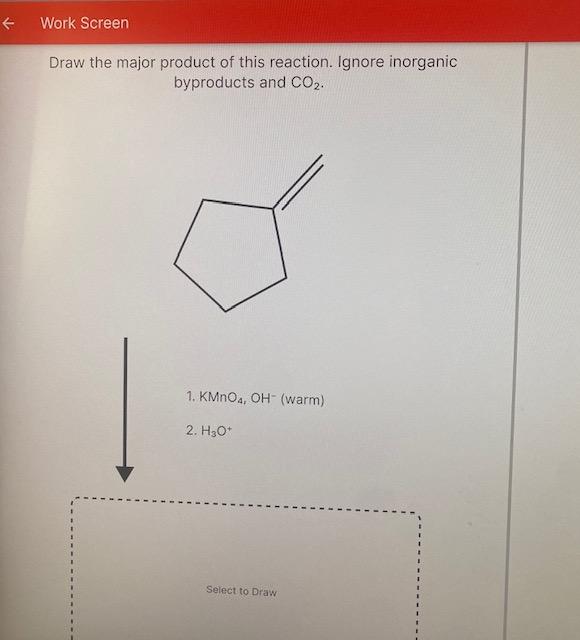
[Solved] . Draw the product of this reaction. Ignore

How do i solve this? Draw the product of this reaction. Ignore
Web Hbr (1 Equiv) Select To Draw Draw The Product Of This Reaction.
Br2 (2 Equiv) Select To Draw Draw The Starting Structure That Would Produce This Product Under These Conditions.
Draw The Starting Structure That Would Lead To The Major Product Shown Under The Provided Conditions.
Finally, The Addition Of H3O+ Will Protonate The Negatively Charged Carbon, Resulting In The Final Product.
Related Post: