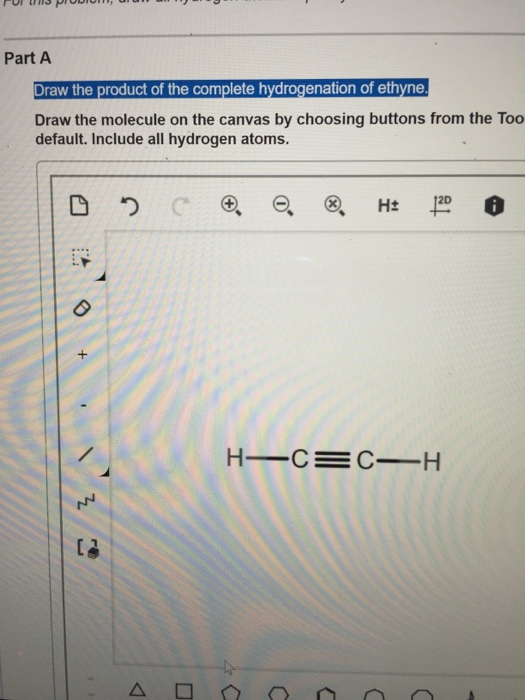
Draw The Product Of The Hydrogenation Of Ethyne
Draw The Product Of The Hydrogenation Of Ethyne - Draw the product of the hydrogenation of ethyne. In ethane it is _______. Answer the complete hydrogenation of ethyne (also known as acetylene) results in the formation of ethane. The single l all hydrogen atoms. Ethene reacts with hydrogen in the presence of a finely divided nickel catalyst at a temperature of about 150°c. The difficulty would be stopping the reduction at the ethylene stage; The single bond is active by default. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. The mechanism of syn addition of the hydrogens. The difficulty would be stopping the reduction at the ethylene stage; Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. View available hint (s) hint 1. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. The difficulty would be stopping the reduction at the ethylene stage; Web chemistry questions and answers. The product of hydrogenation of ethyne (acetylene) is ethane. Web in order to draw the product of the complete hydrogenation of ethyne, we first need to explain the hydrogenation reaction. This problem has been solved! Web part a draw the product of the hydrogenation of ethyne. What is the drawing of this molecule ?? C₂h₂ + 2h₂ → c₂h₆. The product of hydrogenation of ethyne (acetylene) is ethane. Answer the complete hydrogenation of ethyne (also known as acetylene) results in the formation of ethane. In this process, the triple bond between the carbon atoms in ethyne is broken and hydrogen atoms are added, resulting in ethane. Draw the molecule on the canvas by choosing. This is a fairly pointless reaction because ethene is a far more useful compound than ethane! 100% (2 ratings) share share. H −c ≡ c −h +h 2 → h 2c = ch 2. Draw the molecule on the canvas by choosing buttons from the tools (for bonds), atoms, and advanced template toolbars. View available hint(s 12d 6:53 pm a. What is the drawing of this molecule ?? The oxidation number of carbon in ethene is _______; Draw the molecule on the canvas by choosing buttons from the too default. The single bond is active by default. In ethane it is _______. Web in order to draw the product of the complete hydrogenation of ethyne, we first need to explain the hydrogenation reaction. Acetylene → ethylene → ethane. Draw the molecule on the canvas by choosing buttons from the too default. The single bond is active by default. Here’s the best way to solve it. Draw the product of the hydrogenation of ethyne. The difficulty would be stopping the reduction at the ethylene stage; View available hint(s 12d 6:53 pm a 苓 、曲コ )) eng 11/9/2020 48 Web the first one you learned, hydrogen with palladium over carbon, goes all the way to alkane. Web draw the product of the hydrogenation of ethyne? What is the difference between hydration and hydrogenation reactions? Draw the product of the hydrogenation of ethyne. 0.500 mol of ethene reacts with _______ mol of hydrogen. Web hydrogenation in the lab. Web the first one you learned, hydrogen with palladium over carbon, goes all the way to alkane. Thus a chemist might say that ethene reacts with one _______ of hydrogen. This problem has been solved! Acetylene → ethylene → ethane. Web part a draw the product of the hydrogenation of ethyne. Web hydrogenation of ethyne, in the presence of ni, pd or pt and heat, to give ethane as final product.this video is about: Web hydrogenation in the lab. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. H −c ≡ c −h +h 2 → h 2c = ch 2. It is a linear molecule, continue reading. The single l all hydrogen atoms. What is the drawing of this molecule ?? The product of hydrogenation of ethyne (acetylene) is ethane. Web hydrogenation of ethyne can be done by adding hydrogen molecule in the presence of catalyst nickel which can be shown as: But, undoubtedly, experimental protocols exist to do so efficiently. This problem has been solved! Draw the molecule on the canvas by choosing button atoms, and advanced template toolbars. Draw the product of the complete hydrogenation of ethyne. Draw the product of the hydrogenation of ethyne. The mechanism of syn addition of the hydrogens. In this process, the triple bond between the carbon atoms in ethyne is broken and hydrogen atoms are added, resulting in ethane. Ethyne is a hydrocarbon with the formula c2h2. The single bond is active by default. What is the difference between hydration and hydrogenation reactions? H −c ≡ c −h +h 2 → h 2c = ch 2. Organic and biochemistry for the allied health scienceschem 1130. The oxidation number of carbon in ethene is _______;
draw the product of the complete hydrogenation of ethyne. paulartis
draw the product of the complete hydrogenation of ethyne. paulartis
Draw the Product of the Hydrogenation of Ethyne.

HSC Chemistry Module 7 Inquiry Question 3

Catalytic Hydrogenation Reaction of Ethene YouTube

Draw the electron dot structure of ethyne and also draw its structural

draw the product of the complete hydrogenation of ethyne. paulartis
Draw The Product Of The Complete Hydrogenation Of

Ethyne Molecular Orbital Diagram

Hydrogenation of Ethyne, Chemistry Lecture Sabaq.pk YouTube
It Is A Linear Molecule, Continue Reading.
Draw The Molecule On The Canvas By Choosing Buttons From The Too Default.
The Single Bond Is Active By Default.
Draw The Molecule On The Canvas By Choosing Buttons From The Tools (For Bonds), Atoms, And Advanced Template Toolbars.
Related Post: