Draw The Most Important Resonance Contributor Of The Following Structure
Draw The Most Important Resonance Contributor Of The Following Structure - The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets ). Resonance contributors and the resonance hybrid. This is important because neither resonance structure. Web in fact, the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a greater number of atoms: Draw resonance forms and predict the relative contribution of each. Draw the most important resonance contributor of. Interactive 3d display mode ch h.ch draw the. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an. Resonance structures are various forms of. The arrangement of atoms in a molecule or ion is called its. Web in fact, the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a greater number of atoms: Web rules for drawing resonance structures: Draw the most important resonance contributor of. The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets ). Web explain the concept of. Resonance structures for acetate ion. Draw the most important resonance contributor of the following species. Calculate the total number of valence electrons from each atom. Web draw the resonance structures for benzene. 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Begin by watching this video: Draw the most important resonance contributor of the following structure. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Draw the most important resonance contributor of the following species. Resonance structures are various forms of. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. Interactive 3d display mode draw the structure. Web in fact, the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a greater number of atoms: Interactive 3d display mode ch h.ch draw the. Resonance structures for acetate ion. Draw the most important resonance contributor of the following species. Resonance structures are various forms of. Web rules for drawing resonance structures: Interactive 3d display mode ch h.ch draw the. Resonance contributors and the resonance hybrid. Draw the most important resonance contributor of the following species. Begin by watching this video: Interactive 3d display mode draw the structure. Web rules for drawing resonance structures: Resonance contributors and the resonance hybrid. Web rules for drawing resonance structures: Draw resonance forms and predict the relative contribution of each. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Web draw the resonance structures for benzene. Web rules for drawing resonance structures: Interactive 3d display mode ch h.ch draw the. 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. The arrangement of atoms in a molecule or ion is called its. The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets ). Draw resonance forms and predict the relative contribution of each. Calculate the total number of valence electrons from each atom. Resonance contributors and the resonance hybrid. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. Resonance contributors and the resonance hybrid. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. Because of the low hydrogen to carbon ratio in aromatic compounds (note that the h:c ratio in an. Resonance structures for acetate ion. Draw the most important resonance contributor of the following structure. This is important because neither resonance structure. Resonance structures are various forms of. Draw resonance forms and predict the relative contribution of each. The arrangement of atoms in a molecule or ion is called its. Web in fact, the most stable resonance form is the resonance hybrid since it delocalizes the electron density over a greater number of atoms: Interactive 3d display mode draw the structure. Oxygen atoms (3*6) = 18. Begin by watching this video: 1) when you see two different resonance contributors, you are not seeing a chemical reaction! Interactive 3d display mode ch h.ch draw the. Draw the most important resonance contributor of the following species. Web explain the concept of resonance and draw lewis structures representing resonance forms for a given molecule. Web rules for drawing resonance structures: Resonance structures for acetate ion. A each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence. Draw the most important resonance contributor of.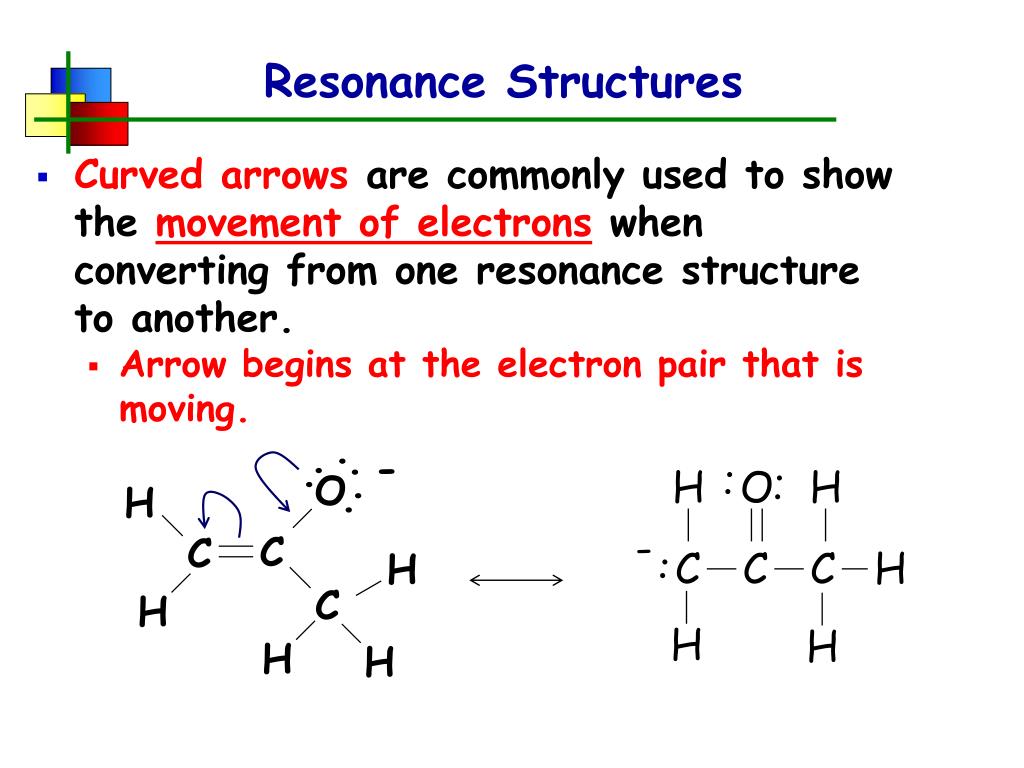
PPT Resonance Structures PowerPoint Presentation, free download ID
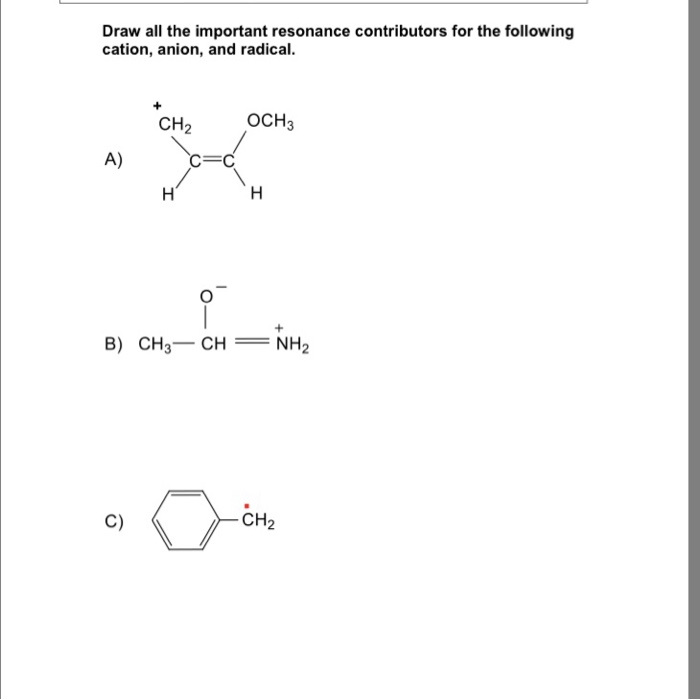
Solved Draw all the important resonance contributors for the
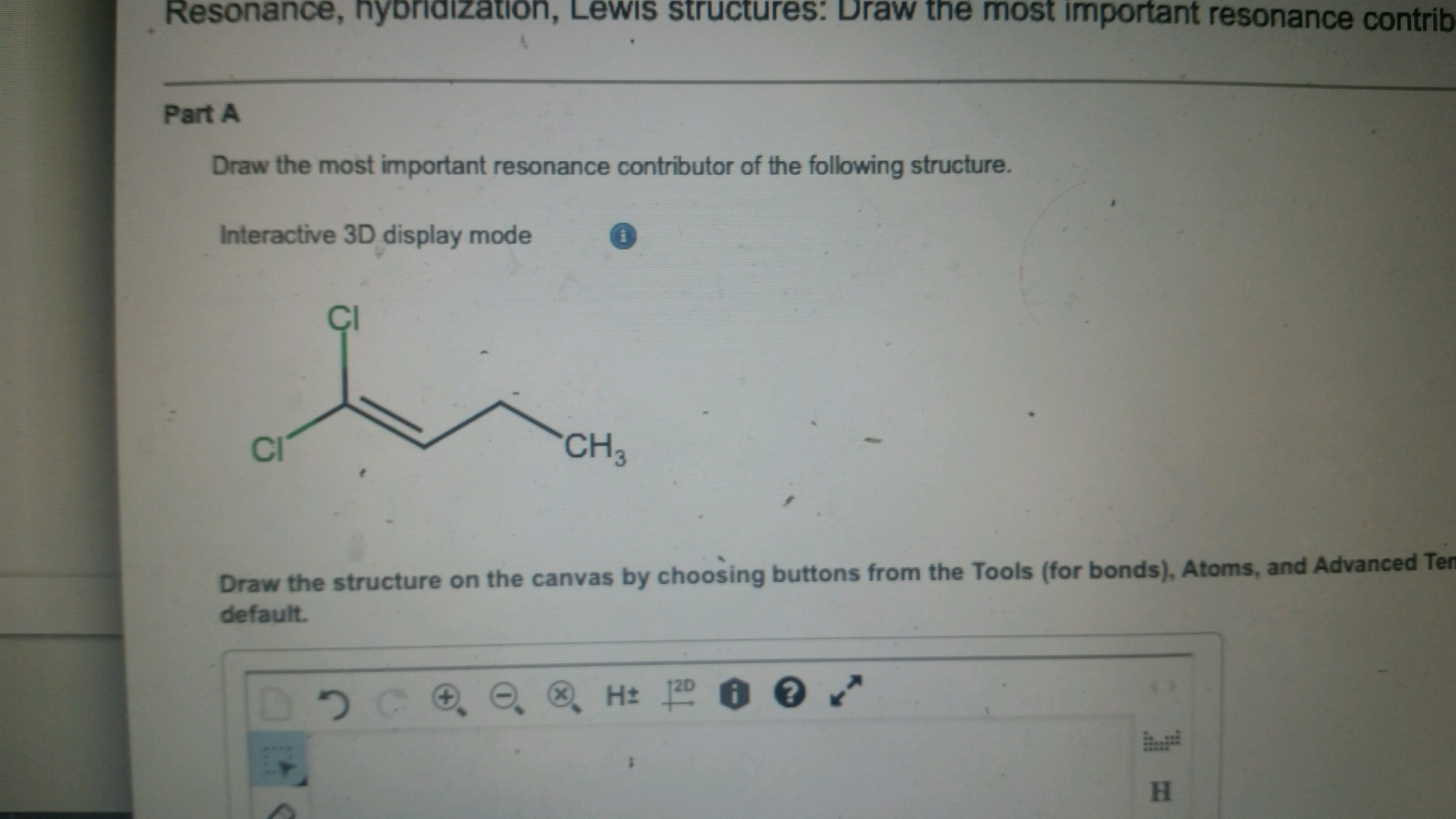
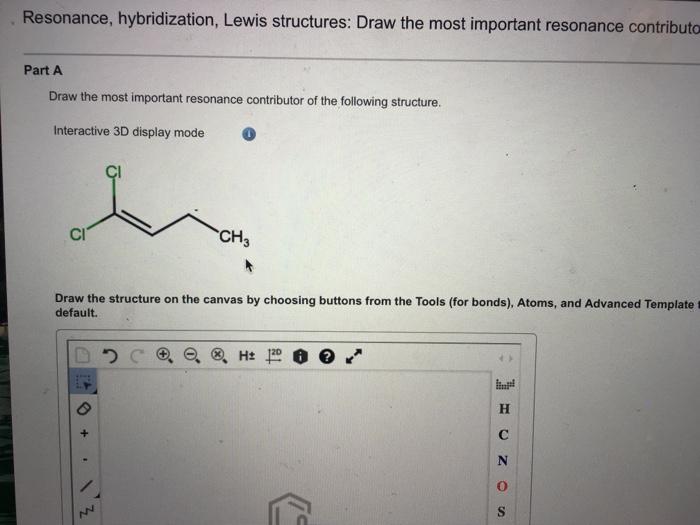
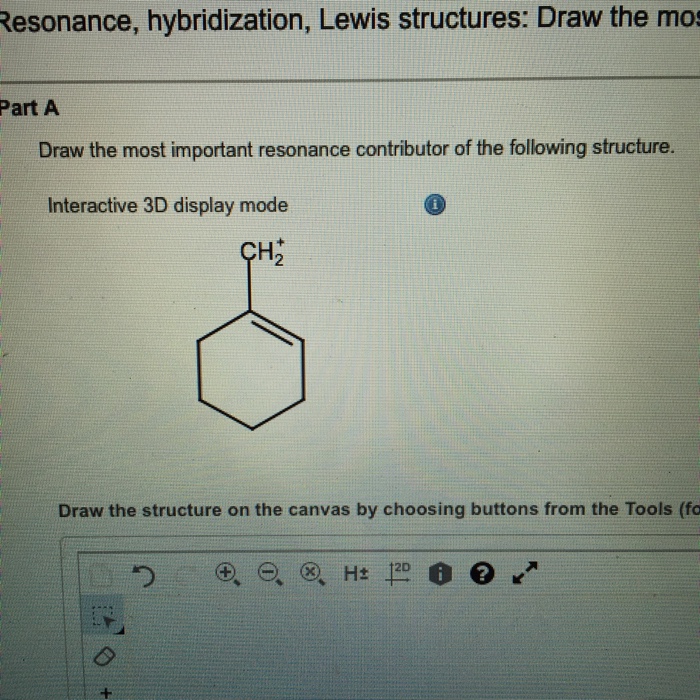
Solved Draw the most important resonance contributor of the
Solved Draw the most important resonance contributor for the

How to Draw Resonance Contributors MCC Organic Chemistry

Solved Draw the most important resonance contributor of the
Solved Draw the most important resonance contributor of the
Solved Draw the most important resonance contributor of the

draw significant resonance structures for the following compound
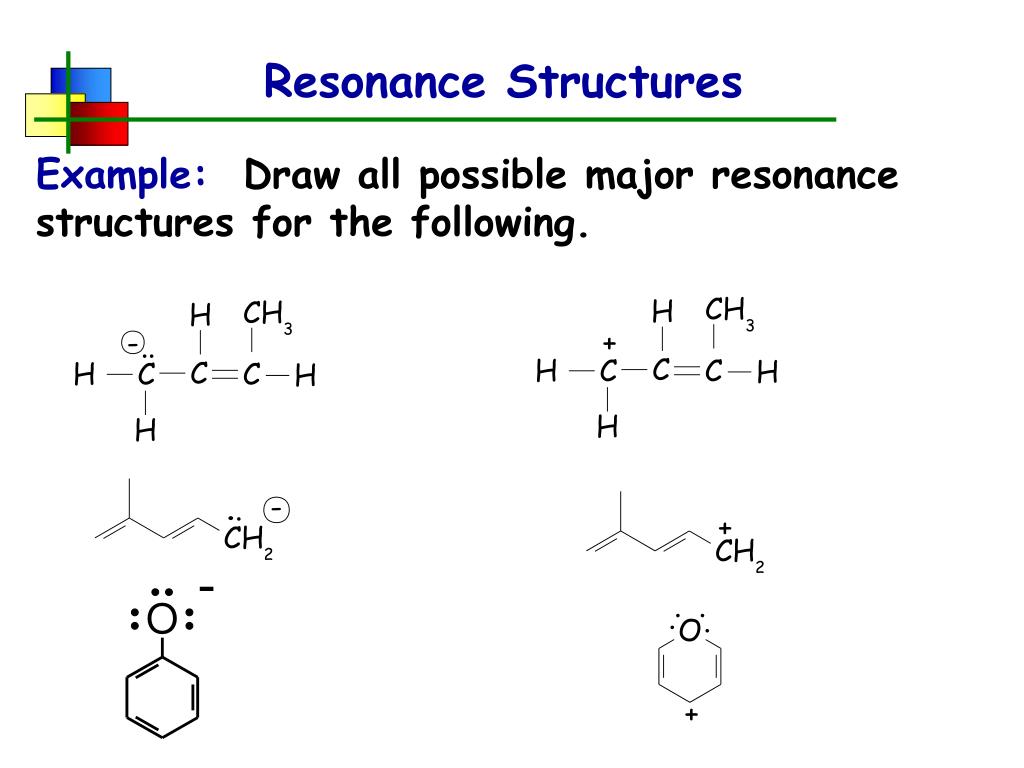
PPT Resonance Structures PowerPoint Presentation, free download ID
Web Draw The Resonance Structures For Benzene.
Resonance Contributors And The Resonance Hybrid.
Calculate The Total Number Of Valence Electrons From Each Atom.
The Most Significant Resonance Contributor Has The Greatest Number Of Full Octets (Or If Applicable, Expanded Octets ).
Related Post: