Draw The Lewis Structure Of Nh3
Draw The Lewis Structure Of Nh3 - Find the total valence electrons in nh3 molecule. Web 2n2 + 3h2 = 2nh3. It also is a good example of a molecule with a trigonal prymidal. 2.5k views 3 years ago. Here, the given molecule is nh3 (ammonia). In order to draw the lewis structure of nh3, first of all you. Please include all nonbonding electrons. This problem has been solved! Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. To draw the nh3 lewis structure, start by counting the total number of valence electrons in the molecule. To begin drawing the nh3 lewis structure, start by counting the. It also is a good example of a molecule with a trigonal prymidal. Nh 3 (ammonia) is a commonly tested lewis structure. Please include all nonbonding electrons. Web to illustrate this method, let’s calculate the formal charge on the atoms in ammonia (nh3) whose lewis electron structure is as. 2.5k views 3 years ago. Web there are three single bonds and one lone pair of electrons in the nh3 molecule. Nitrogen contributes 5 valence electrons, while each hydrogen. This problem has been solved! Draw the lewis structure for nh3. A neutral nitrogen atom has five valence. This will help you understand the molecule’s electronic structure. Web drawing lewis structures for molecules with one central atom: Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Draw the lewis structure for nh3. To draw the nh3 lewis structure, start by counting the total number of valence electrons in the molecule. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. This problem has been solved! How to draw nh3 lewis dot structure? Count the total number of valence electrons. Web 2n2 + 3h2 = 2nh3. Starting from this structure, complete the lewis structure that follows the octet rule on all. To draw the nh3 lewis structure, start by counting the total number of valence electrons in the molecule. Nitrogen contributes 5 valence electrons, while each hydrogen. To begin drawing the nh3 lewis structure, start by counting the. Nh 3 (ammonia) is a commonly tested lewis structure. In order to find the total valence electrons in nh3 molecule,. In order to draw the lewis structure of nh3, first of all you. Count the total number of valence electrons. Starting from this structure, complete the lewis structure that follows the octet rule on all. It also is a good example of a molecule with a trigonal prymidal. Web the lewis structure for ammonia (nh₃) shown below is incorrect. To draw the nh3 lewis structure, start by counting the total number of valence electrons in the molecule. In order to draw the lewis structure of nh3, first of all you. It has a molecular geometry. Web the lewis structure for ammonia (nh₃) shown below is incorrect. It also is a good example of a molecule with a trigonal prymidal. Web steps of drawing nh3 lewis structure. Craig beals shows how to draw the lewis structure for ammonia. This problem has been solved! Craig beals shows how to draw the lewis structure for ammonia. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Web ammonia (nh 3) is a commonly tested lewis structure due to it's widespread use in agriculture as a fertilizer. Web steps of drawing nh3 lewis structure. Draw the lewis structure for nh3. This will help you understand the molecule’s electronic structure. It has a molecular geometry of trigonal pyramidal which also looks like a. Web drawing the lewis structure for nh 3. Web drawing lewis structures for molecules with one central atom: How to draw nh3 lewis dot structure? To begin drawing the nh3 lewis structure, start by counting the. Web steps of drawing nh3 lewis structure. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. It's not particularly difficult but is an important structure. Web there are three single bonds and one lone pair of electrons in the nh3 molecule. Web 2n2 + 3h2 = 2nh3. Web drawing lewis structures for molecules with one central atom: How to draw nh3 lewis dot structure? Count the total number of valence electrons. Craig beals shows how to draw the lewis structure for ammonia. Please include all nonbonding electrons. It also is a good example of a molecule with a trigonal prymidal. Web to draw the nh3 lewis structure (ammonia) involves a few straightforward steps. Find the total valence electrons in nh3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a. Here, the given molecule is nh3 (ammonia).
Lewis Structure NH3 plus dipoles, shape, angles and formal charge

NH3 Molecular Geometry Science Education and Tutorials

NH3 (Ammonia) Lewis Structure Drawing Method, Molecular Geometry
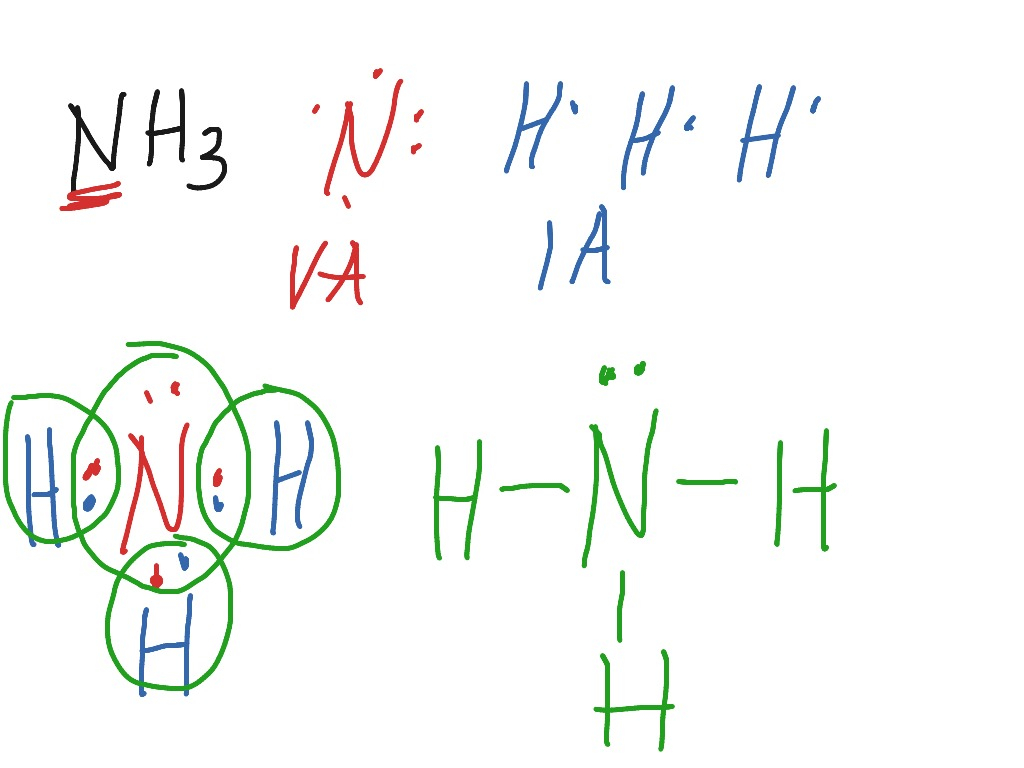
Lewis Dot Diagram For Nh3 exatin.info

The Nh3 Lewis Dot Structure Understanding The Basics vrogue.co

NH3 Lewis Structure How to Draw the Dot Structure for NH3 YouTube
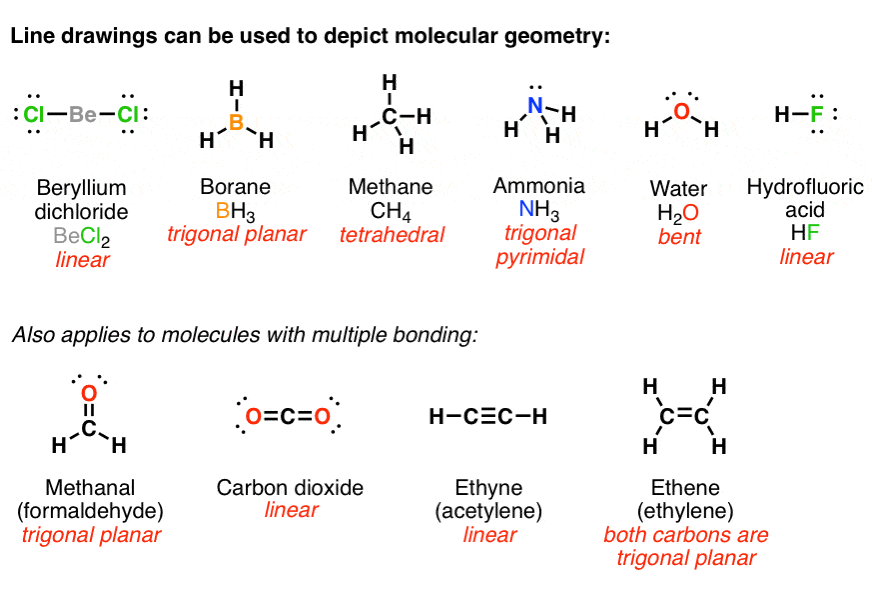
Draw The Lewis Structure For NH3

Lewis Structures How to draw NH3 YouTube
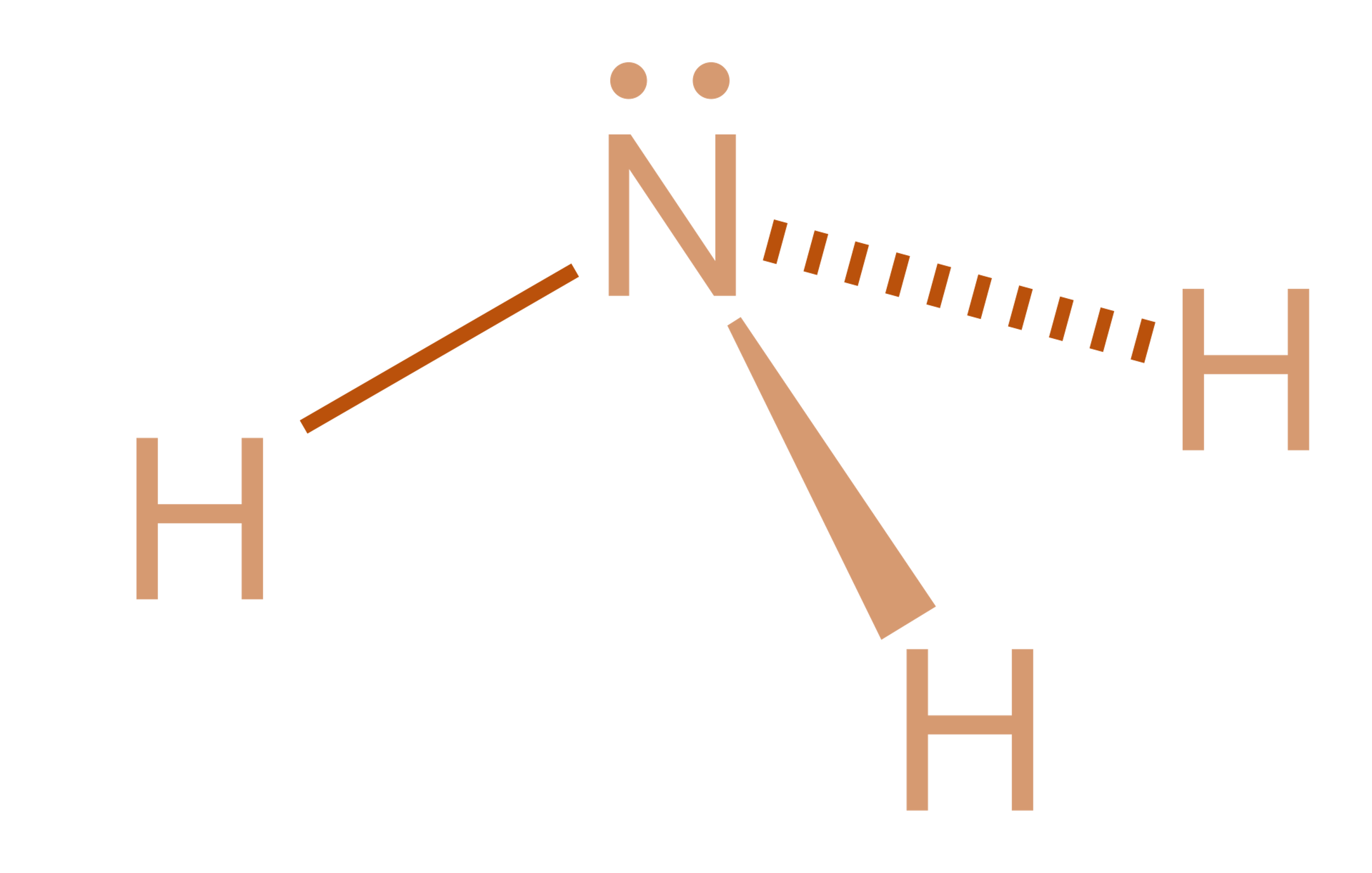
Nh3 Lewis Structure Molecular Geometry

NH3 Lewis Structure 5 Easy Steps to Draw, Hybridization
The Lewis Structure Of Ammonia, N H 3, Would Be Three Hydrogen Atoms Bonded To A Nitrogen Atom In The Middle, With A Lone Pair Of Electrons On.
Nitrogen Contributes 5 Valence Electrons, While Each Hydrogen.
In Order To Find The Total Valence Electrons In Nh3 Molecule,.
A Neutral Nitrogen Atom Has Five Valence.
Related Post: