Draw The Lewis Structure Of Co3 2
Draw The Lewis Structure Of Co3 2 - Then, determine the formal charges on the atoms and minimize them by converting any available lone pairs to chemical bonds. Drawing lewis structures for bf3, pf3 and brf3; There are 2 single bonds and 1 double bond between the carbon atom (c) and each oxygen atom (o). I also go over the resonance, hybridization, shape and bond angle. To envision this, we can start by drawing the average of the two structures. Each of the singly bonded. It consists of one carbon atom (c) bonded to three oxygen atoms (o) through double bonds, with one of the oxygen atoms also carrying a negative charge. Using formal charges to determine how many bonds to make, a different perspective. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web put one electron pair in each bond 4. Web drawing lewis structures for molecules with one central atom: 3.5) c) determine the polarity of the co bond. Each carbon oxygen bond can be thought of as 1.333 bonds. We start with a valid lewis structure and then follow these general. Then, determine the formal charges on the atoms and minimize them by converting any available lone pairs to chemical bonds. We start with a valid lewis structure and then follow these general. Move electrons so all atoms (esp. Find more chemistry widgets in wolfram|alpha. Let’s consider the lewis structure of the carbonate ion, co32‐. Hence, lewis structure is also commonly called electron dot structure. We start with a valid lewis structure and then follow these general. Web drawing lewis structures for molecules with one central atom: Therefore, the true structure of hno 2 looks more like the structure on the left. Breaking the octet rule ; Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. Let’s consider the lewis structure of the carbonate ion, co32‐. Fill outer atoms (o) with electrons first. Each carbon oxygen bond can be thought of as 1.333 bonds. The centre one) has a full octet. The centre one) has a full octet. The average of a double bond and 2 single bonds. Drawing lewis structures for bf3, pf3 and brf3; Web drawing lewis structures for molecules with one central atom: Using formal charges to determine how many bonds to make, a different perspective. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Move electrons so all atoms (esp. Each carbon oxygen bond can be thought of as 1.333 bonds. Each of the singly bonded. To envision this, we can start by drawing the average of the two structures. The average of a double bond and 2 single bonds. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. It consists of one carbon atom (c) bonded to three oxygen atoms (o) through double bonds, with one of the oxygen. The correct lewis structure for this ion. Hence, lewis structure is also commonly called electron dot structure. Has one carbon‐oxygen double bond, and two carbon‐oxygen single bonds. There are three different possible resonance structures from carbonate. Web put one electron pair in each bond 4. Find total number of electrons of the valance shells of carbon and oxygen atoms; The correct lewis structure for this ion. Web since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet rule. Count the total number of valence. Each co atom also has one lone pair of. Calculate the total number of valence electrons. It consists of one carbon atom (c) bonded to three oxygen atoms (o) through double bonds, with one of the oxygen atoms also carrying a negative charge. The average of a double bond and 2 single bonds. Find more chemistry widgets in wolfram|alpha. Web since carbon is located in period 2 it does. The average of a double bond and 2 single bonds. Let’s consider the lewis structure of the carbonate ion, co32‐. Web drawing lewis structures for molecules with one central atom: I also go over the resonance, hybridization, shape and bond angle. Therefore, the true structure of hno 2 looks more like the structure on the left. To envision this, we can start by drawing the average of the two structures. Determine the total number of valence electrons in the molecule by adding up the valence electrons of each atom. Find more chemistry widgets in wolfram|alpha. Each carbon oxygen bond can be thought of as 1.333 bonds. Each of the singly bonded. Each co atom also has one lone pair of electrons. Breaking the octet rule ; There are 2 single bonds and 1 double bond between the carbon atom (c) and each oxygen atom (o). The centre one) has a full octet. Move electrons so all atoms (esp. Web since carbon is located in period 2 it does not have access to the d sublevel and must adhere to the octet rule.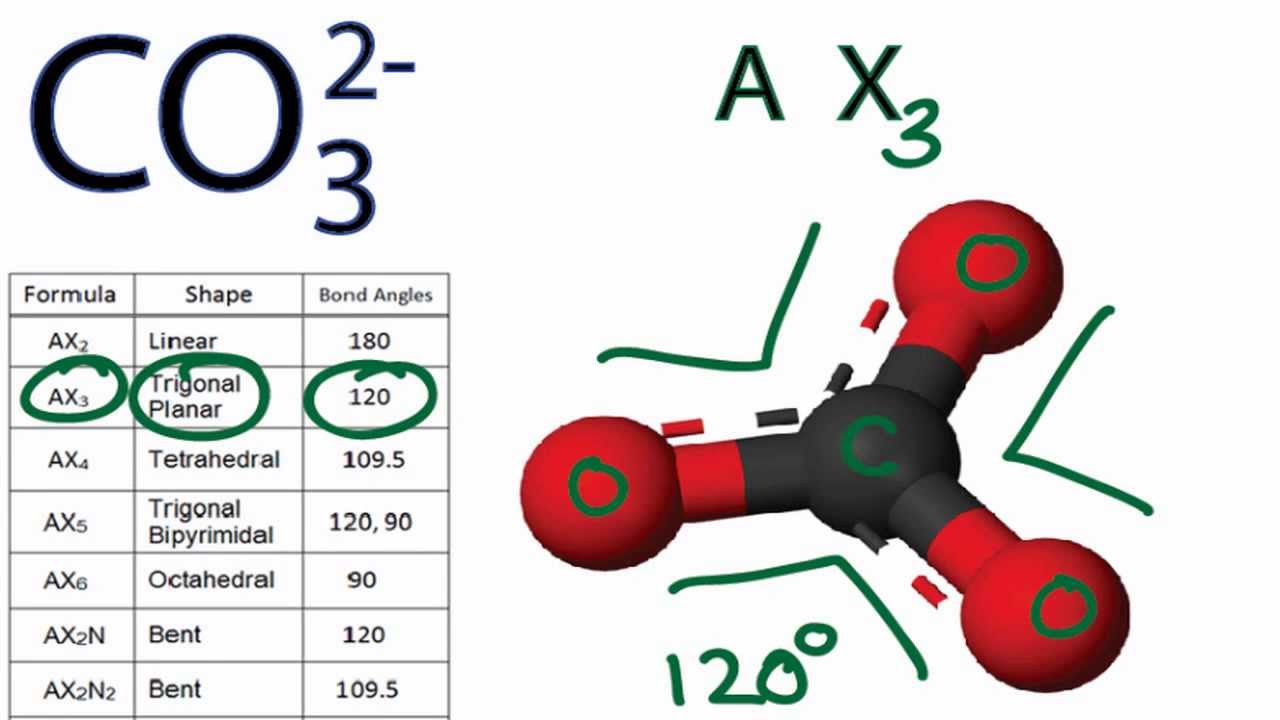
CO3 2 Molecular Geometry / Shape and Bond Angles YouTube
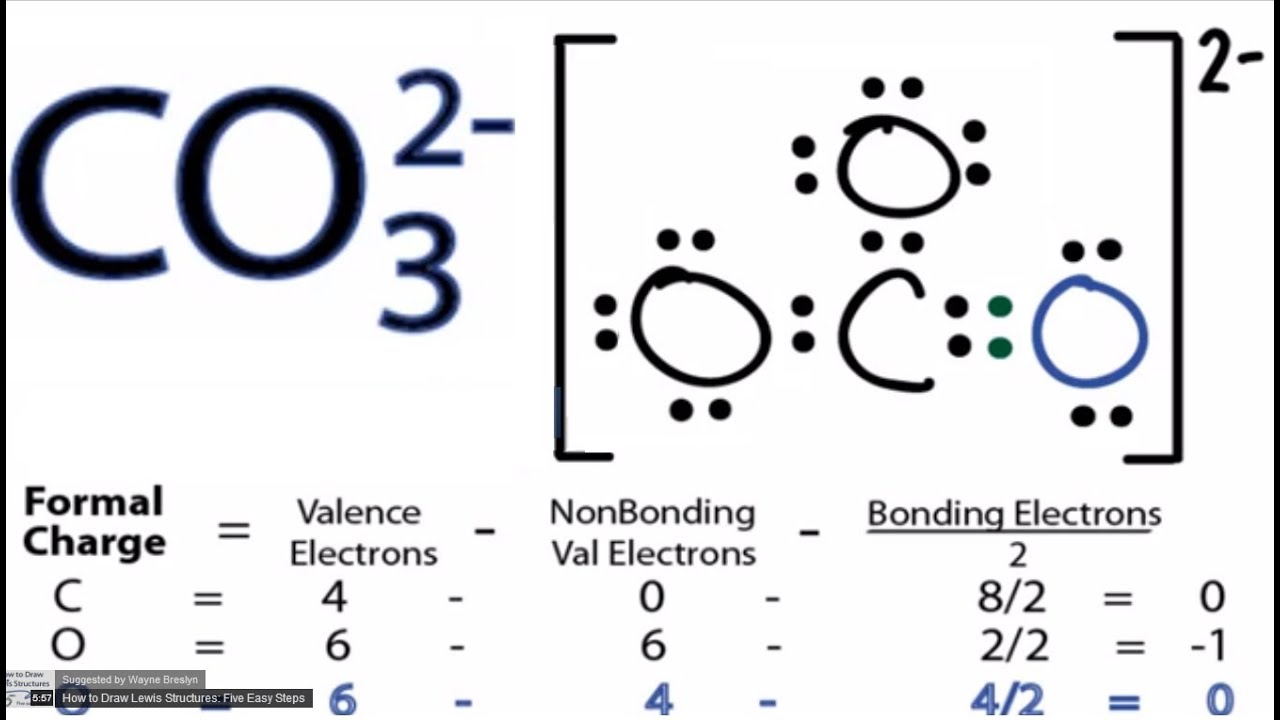
CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2

How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube

Draw the Lewis structure for CO3^2
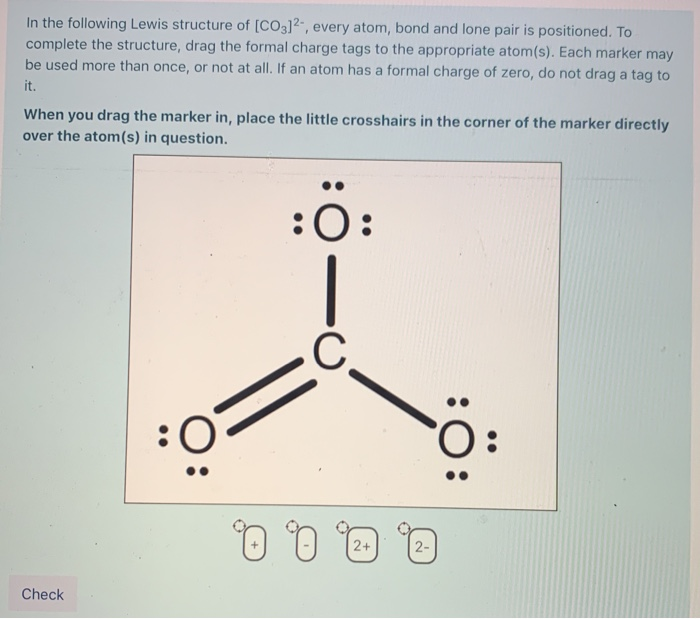
Solved In the following Lewis structure of [CO3)2, every
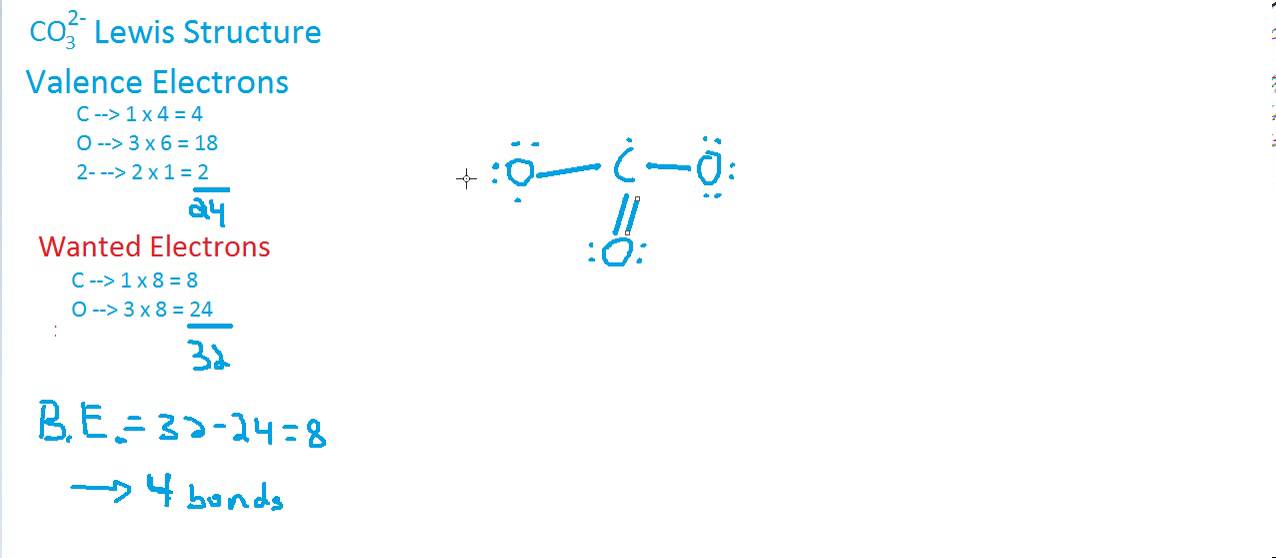
CO3 2 Lewis Structure & Geometry YouTube

Draw Lewis dot structure for CO3 2( carbonate ion) YouTube

Lewis structure of CO3 2 ion YouTube

How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry

Lewis Structure for CO3 2 (Carbonate ion) YouTube
Web Put One Electron Pair In Each Bond 4.
Valence Electrons Are The Electrons In The Outermost Shell Of An Atom That Participate In Chemical Bonding.
It Consists Of One Carbon Atom (C) Bonded To Three Oxygen Atoms (O) Through Double Bonds, With One Of The Oxygen Atoms Also Carrying A Negative Charge.
May I Recommend A Video.
Related Post: