Draw The Lewis Structure Of Co2
Draw The Lewis Structure Of Co2 - Some molecules must have multiple covalent bonds between atoms to satisfy the octet rule. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. Two double bonds connect the carbon and oxygen atoms in the lewis structure. The carbon atom is sp hybridized and oxygen atoms are sp2, making the overall. Still, 12 electrons are remaining. Carbon has 4 valence electrons, while each oxygen atom has 6 valence electrons. Draw the lewis dot structure for carbon dioxide below. In the co 2 lewis structure carbon is the least electronegative element. Next, we need to arrange the atoms in the molecule. A lewis structure shows the bonding and nonbonding electrons around individual atoms in a molecule. The hybridization of co2 is sp. Web drawing the lewis structure for co 2. Web carbon needs two double bonds, one to each of the two oxygens, to complete its octet. Carbon is the central atom in co2, with each oxygen atom. Draw the lewis dot structure for the hydrogen atom. A lewis structure shows the bonding and nonbonding electrons around individual atoms in a molecule. Carbon has 4 valence electrons, while each oxygen atom has 6 valence electrons. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule. A lewis structure shows the bonding and nonbonding electrons around individual atoms in a molecule. See the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding. Web this widget gets the lewis structure of chemical compounds. Web this is the complete lewis structure of co 2. Web carbon dioxide (co 2) lewis structure. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. To further understand the molecular geometry of co2, let us quickly go through its. In the co 2 lewis structure carbon is the least electronegative element. Web i quickly take you through how to draw the lewis structure of co2 (carbon dioxide). There. Web to draw the lewis structure of co2, we first need to determine the number of valence electrons in each atom. Therefore it is put in the center of the dot structure. Next, we need to arrange the atoms in the molecule. Web set the atom electronegativities of atom a, b, and c. Web i quickly take you through how. Web to draw the lewis structure of co2, we first need to determine the number of valence electrons in each atom. Web i quickly take you through how to draw the lewis structure of co2 (carbon dioxide). Web set the atom electronegativities of atom a, b, and c. Web for lewis structure of co2, you will now have two oxygen. Be sure to have the correct number of electrons. Web i quickly take you through how to draw the lewis structure of co2 (carbon dioxide). Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone. Find more chemistry widgets in wolfram|alpha. Web i quickly take you through how to draw the lewis structure of co2 (carbon dioxide). Web for lewis structure of co2, you will now have two oxygen atoms forming double bonds with a carbon atom. Be sure to have the correct number of electrons. In the co 2 lewis structure carbon is the. Connect the atoms to each other with single bonds to form a “skeleton structure.”. In the earth's atomsphere it is considered a greenhouse gas. Find more chemistry widgets in wolfram|alpha. Web lewis structures (also known as lewis dot diagrams, electron dot diagrams,lewis dot formula lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of. Shape of co 2 is linear. Web this chemistry video explains how to draw the lewis structure of co2 also known as carbon dioxide. Web carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon. Web set the atom electronegativities of atom a, b, and c. Two double bonds connect the carbon and oxygen atoms in. Shape of co 2 is linear. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web this chemistry video explains how to draw the lewis structure of co2 also known as carbon dioxide. Here, the given molecule is co2 (carbon dioxide). Web this is the complete lewis structure of co 2. Web for lewis structure of co2, you will now have two oxygen atoms forming double bonds with a carbon atom. Web to draw the lewis structure of co2, we first need to determine the number of valence electrons in each atom. Since hydrogen is in group i it has one (1) valence electron in. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Therefore it is put in the center of the dot structure. Next, we need to arrange the atoms in the molecule. Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Web this type of lewis dot structure is represented by an atomic symbol and a series of dots. In the earth's atomsphere it is considered a greenhouse gas. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Web carbon dioxide is a colourless, odourless, incombustible gas produced by the combustion of carbon.
CO2 Lewis Structure How to Draw or Write the Lewis Dot Structure for
:max_bytes(150000):strip_icc()/CO2LewisStructure-591c94063df78cf5fadfde77.png)
CO2 Molecule Lewis Structure

CO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide

What is the Lewis Dot structure for CO2 (Carbon dioxide)?

What is the Lewis Dot structure for CO2 (Carbon dioxide)?
![Lewis Structure of CO2 [with video and free study guide]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/CO2-lewis-puzzle-1024x554.jpg)
Lewis Structure of CO2 [with video and free study guide]

How to draw electron dot structure of CO2 How to draw Lewis dot
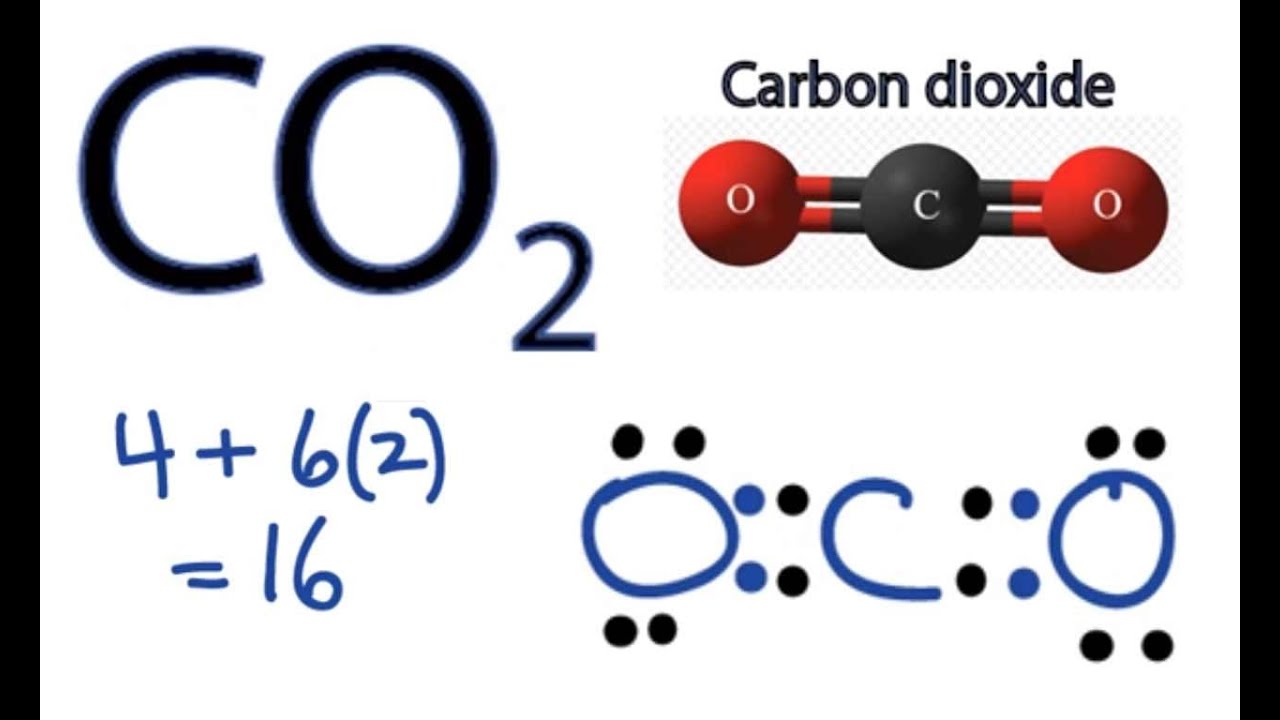
CO2 Lewis Structure How to Draw the Dot Structure for Carbon Dioxide

CO2 Lewis Structure ,Valence Electrons, Formal Charge ,Polar or
Draw The Lewis Dot Structure For Carbon Dioxide Below.
Two Oxygen Atoms Are Present At The Terminals, Where They Share Electrons And Form Bonds With The Central Carbon Atom.
Web Carbon Needs Two Double Bonds, One To Each Of The Two Oxygens, To Complete Its Octet.
In Order To Draw The Lewis Structure Of Co2, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Co2 Molecule.
Related Post: