Draw The Lewis Structure Of Cl2
Draw The Lewis Structure Of Cl2 - 18k views 3 years ago. Draw the lewis structure of ch2cl2. (valence electrons are the number of electrons present in the outermost shell of an atom). Here, the given molecule is cl2 (chlorine). Web 6 steps to draw the lewis structure of cl2 step #1: How to draw a lewis structure. Let’s discuss each step in more detail. How to draw lewis structure for cl 2; Web in order to draw the lewis structure of cl2o2, first of all you have to find the total number of valence electrons present in the cl2o2 molecule. Web use these steps to correctly draw the cl 2 lewis structure: How to draw lewis structure for cl 2; Web steps to draw the lewis structure of cl2. Drawing lewis structures for bf3, pf3 and brf3; Web draw lewis structures depicting the bonding in simple molecules. So, this lewis structure is a very simple. This widget gets the lewis structure of chemical compounds. Draw the lewis structure of o2. Web draw lewis structures for covalent compounds. Cl 2 lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. Web use these steps to correctly draw the cl 2 lewis structure: Find more chemistry widgets in wolfram|alpha. Web draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond. Web the lewis structure of cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on. How to draw lewis structure for cl 2; Draw the lewis structure of o2. 166k views 10 years ago. (note that we denote ions with brackets around the. Cl 2 lewis structure consists of two chlorine atoms linked by a single bond with three lone pairs on each chlorine. All atoms must have 8 electrons 2.only use the valence electrons that atom had to start with. And what type of bond is this. Web draw a plausible lewis electron structure for a compound with the molecular formula cl 3 po. Draw the lewis structure of ch2cl2. Web draw lewis structures depicting the bonding in simple molecules. Send feedback | visit wolfram|alpha. How to draw a lewis structure. Web in order to draw the lewis structure of cl2o2, first of all you have to find the total number of valence electrons present in the cl2o2 molecule. Crack cds exams with india's super teachers. All atoms must have 8 electrons 2.only use the valence electrons that atom had. It is very easy to draw the cl 2 lewis structure. For the cl 2 lewis structure there are a. Web the lewis structure of cl 2 consists of two chlorine atoms connected by a single bond with three lone pairs on each chlorine. Molecular geometry of cl 2; Cl 2 is a green, poisonous gas. So, this lewis structure is a very simple. How to draw a lewis structure. Choose a suitable central atom for the compound. For the cl 2 lewis structure there are a. #2 mark lone pairs on the atoms. How to draw lewis structure for cl 2; All atoms must have 8 electrons 2.only use the valence electrons that atom had to start with. This is done by adding the valence shell electrons of all the constituent atoms. Choose a suitable central atom for the compound. So, let’s calculate this first. Choose a suitable central atom for the compound. All atoms must have 8 electrons 2.only use the valence electrons that atom had to start with. Count the total number of valence shell electrons on the molecule. #1 first draw a rough sketch. Web draw a plausible lewis electron structure for a compound with the molecular formula cl 3 po. In order to find the total valence electrons in a cl2 (chlorine) molecule, first of all you should know the valence electrons present in a single chlorine atom. 18k views 3 years ago. #1 first draw a rough sketch. Web use these steps to correctly draw the cl 2 lewis structure: #2 mark lone pairs on the atoms. Web draw lewis structures depicting the bonding in simple molecules. (valence electrons are the number of electrons present in the outermost shell of an atom). Send feedback | visit wolfram|alpha. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. This is done by adding the valence shell electrons of all the constituent atoms. So, this lewis structure is a very simple. Using the periodic table to draw lewis dot structures. Find more chemistry widgets in wolfram|alpha. Breaking the octet rule ; Drawing lewis structures for bf3, pf3 and brf3; And what type of bond is this.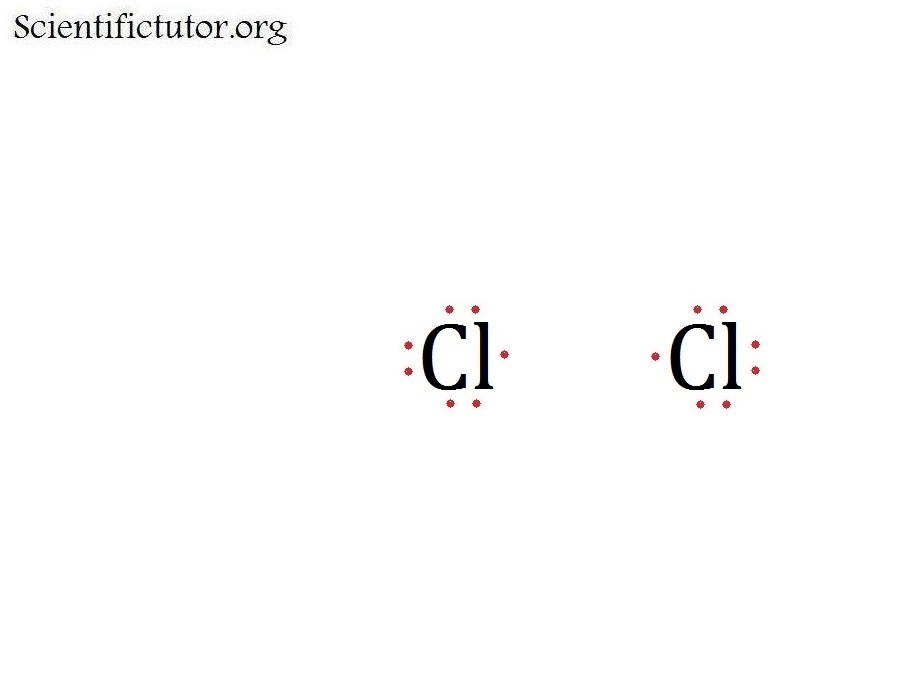
Covalent Bonds in Electron Dot Structures (Lewis Structures
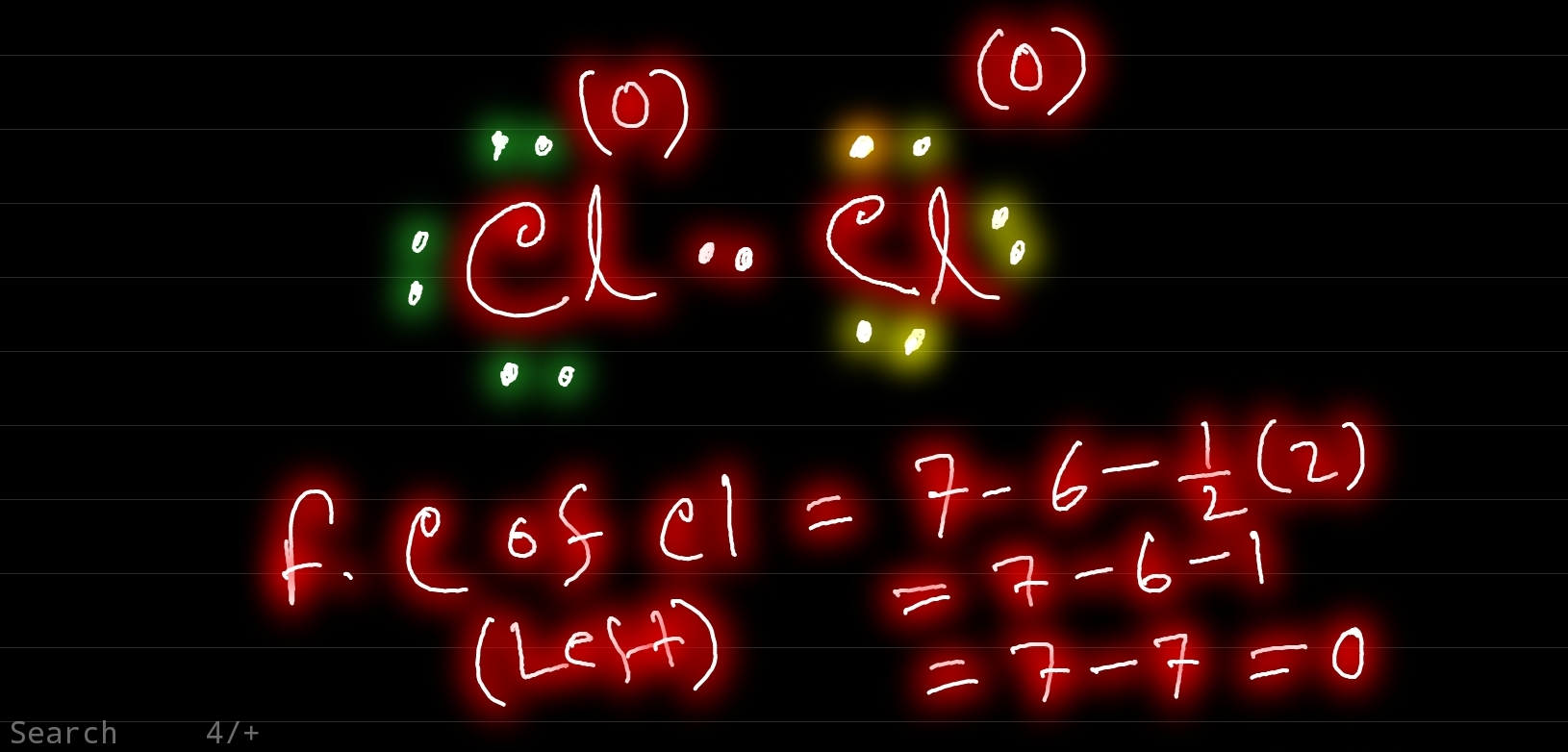
Cl2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar

Cl2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
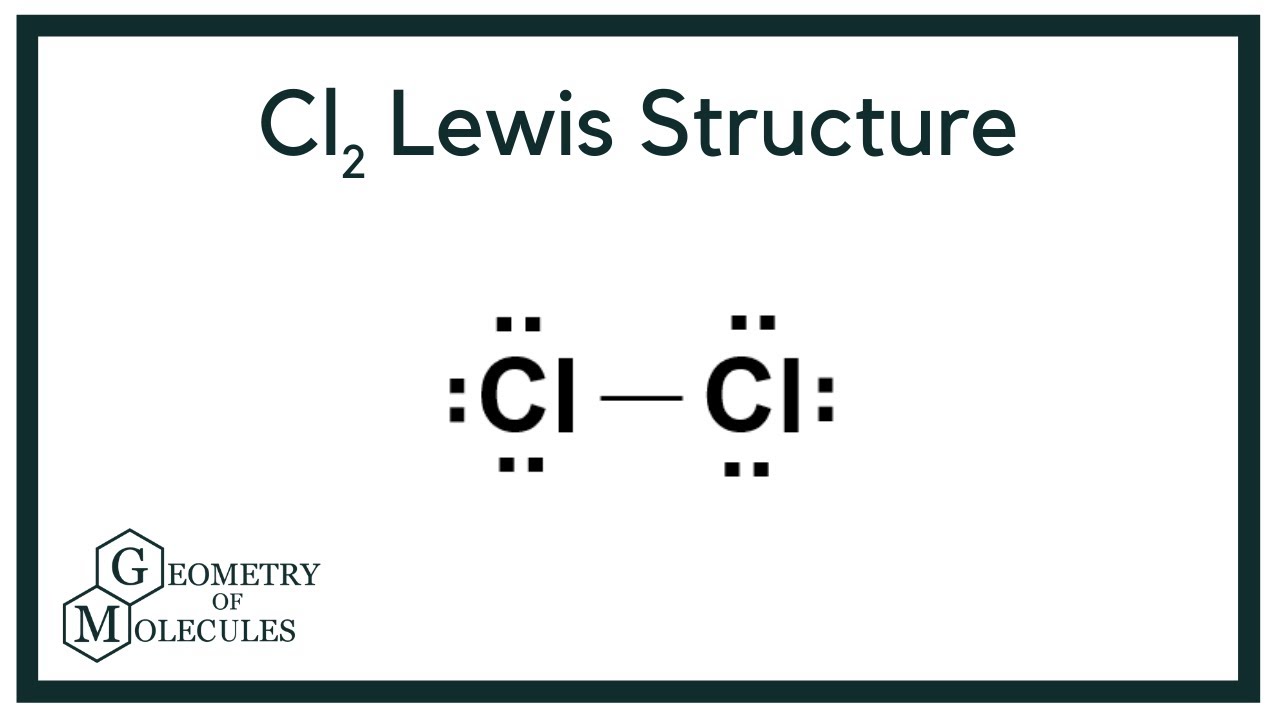
Cl2 Lewis Structure (Dichlorine) YouTube
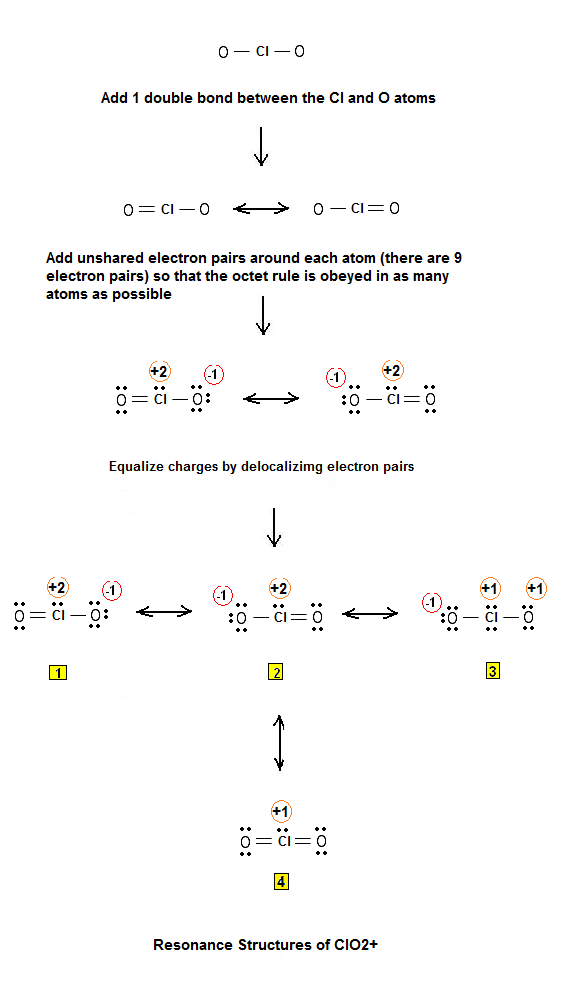
Lewis ElectronDot Structure for Chlorine Dioxide Ion (ClO2

【2 Steps】Cl2 Lewis Structure Lewis Dot Structure for Chlorine(Cl
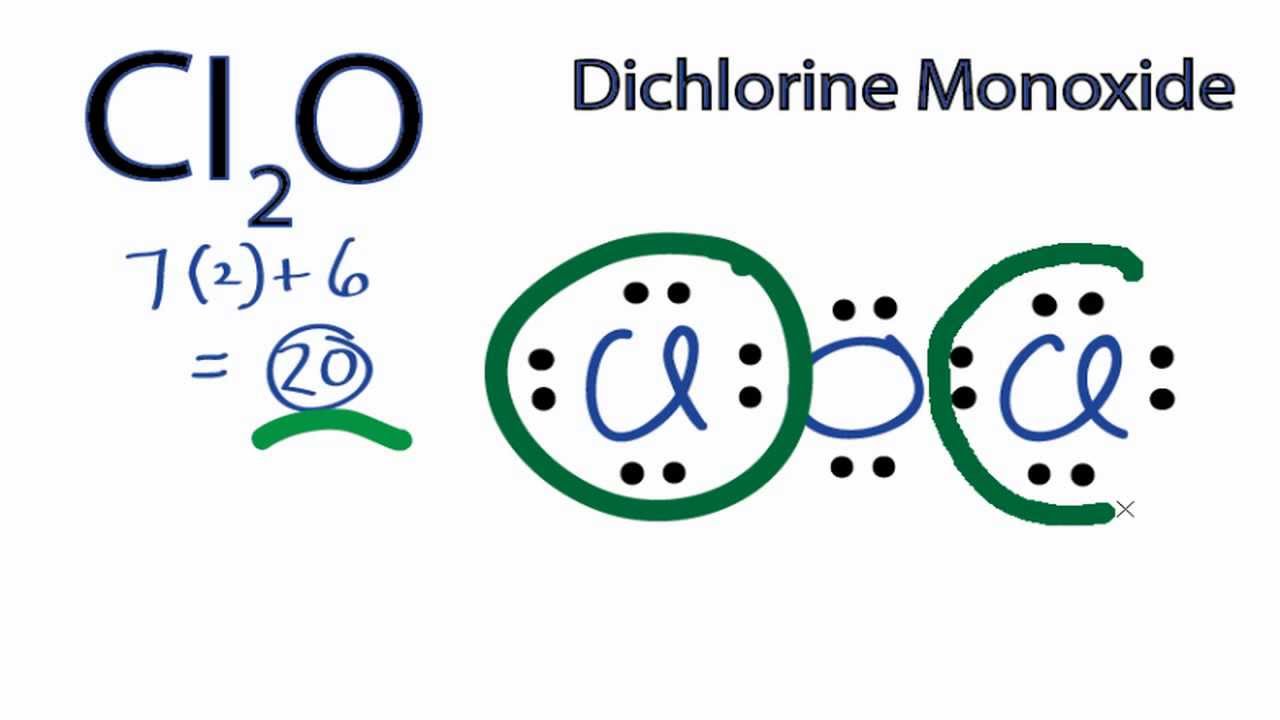
Estructura De Lewis Cl2 farez
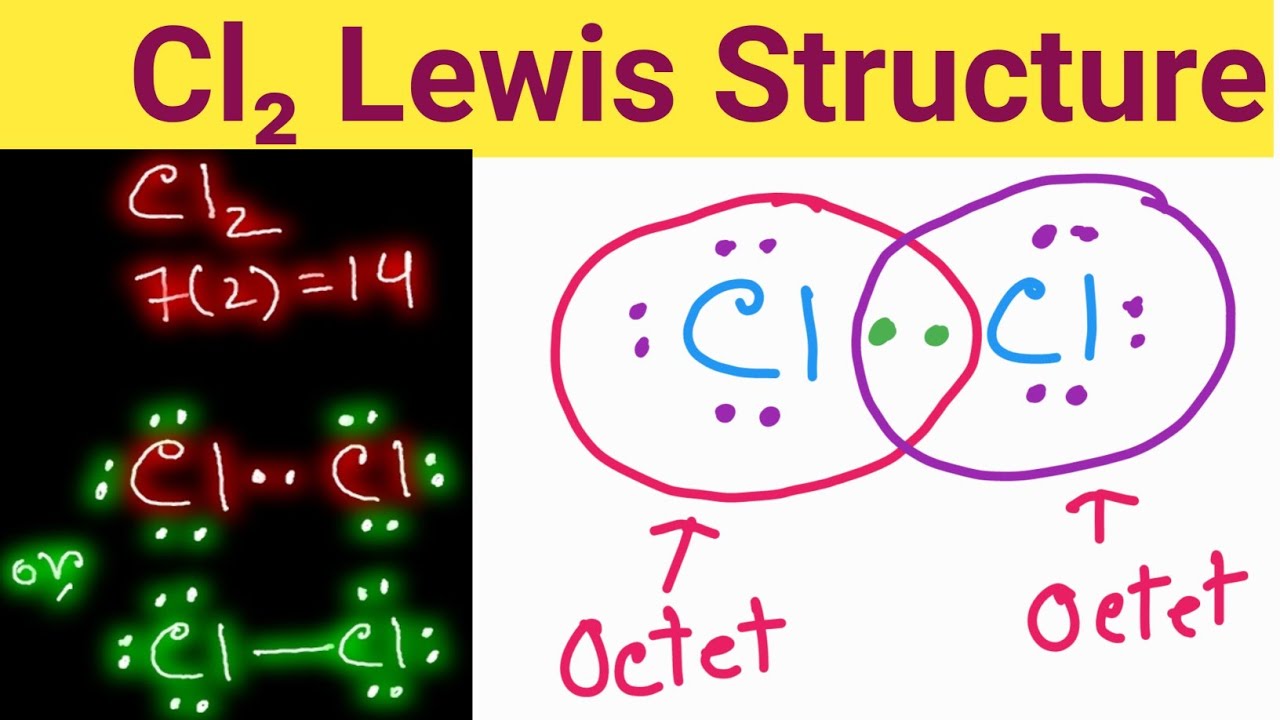
Cl2 Lewis Structure Lewis Dot Structure for Cl2 Lewis Structure of
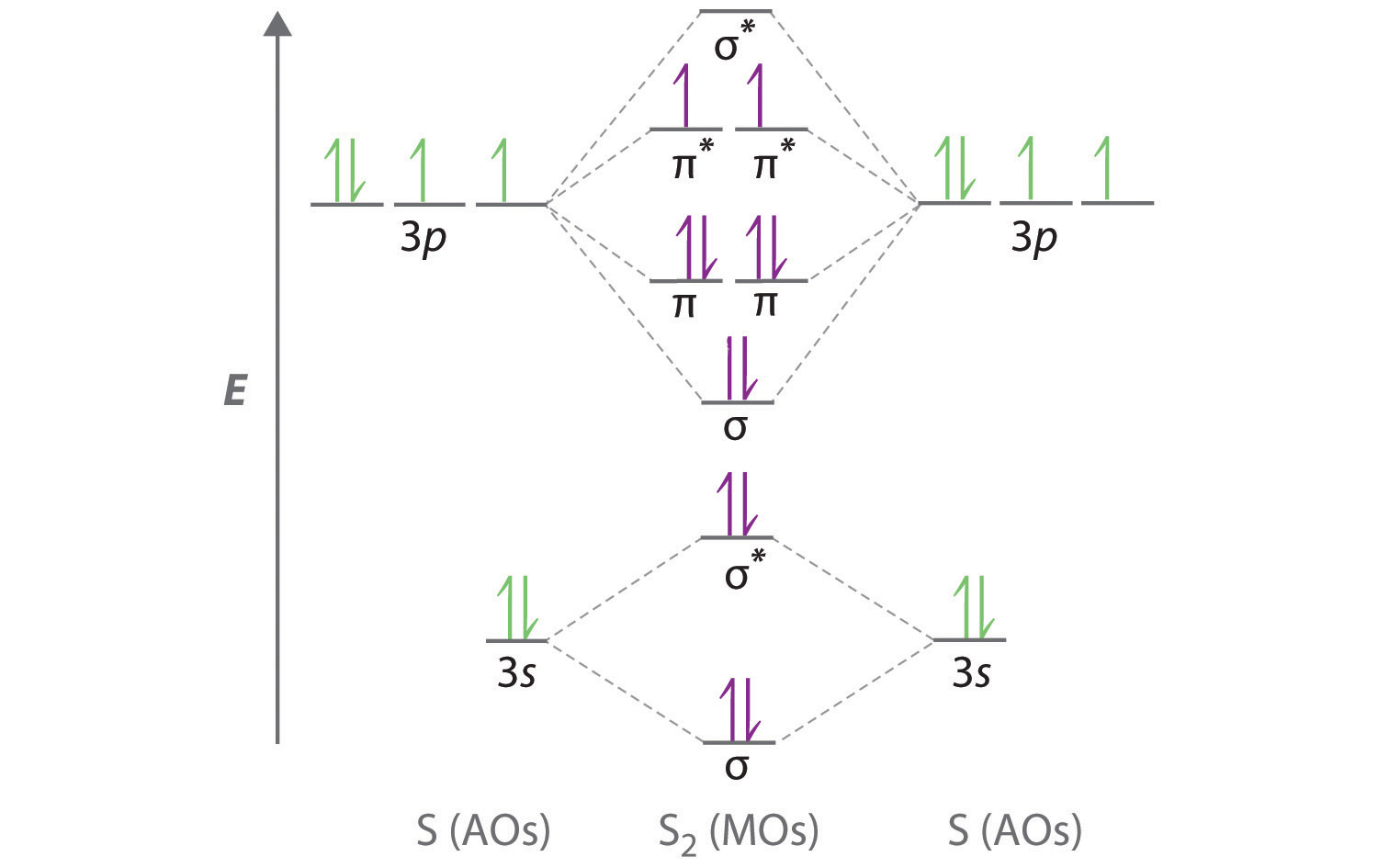
Molecular Orbital Diagram For Cl2
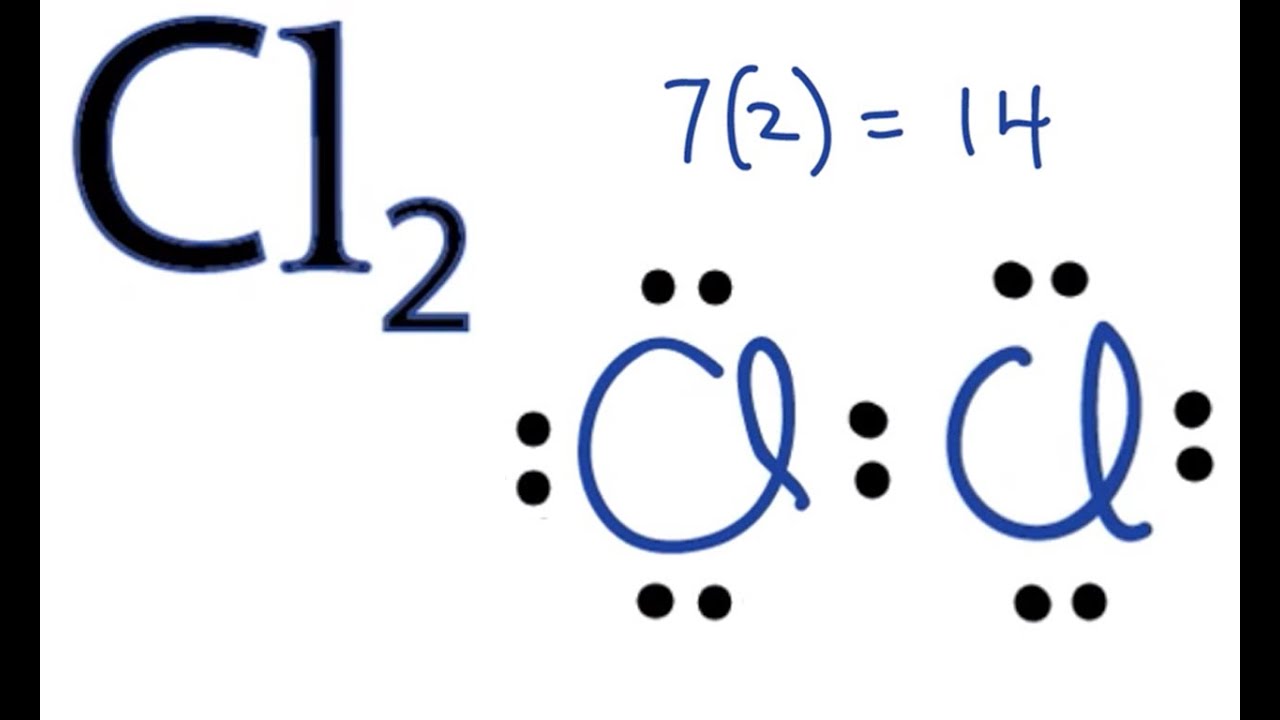
Cl2 Lewis Structure How to Draw the Dot Structure for Cl2 YouTube
How To Draw Lewis Structure For Cl 2;
Cl 2 Is A Green, Poisonous Gas.
Using Lewis Structures To Show Valence Electrons.
First, Determine The Total Number Of Valence Electrons.
Related Post: