Draw The Lewis Structure For The Iodide Pentafluoride Molecule
Draw The Lewis Structure For The Iodide Pentafluoride Molecule - In order to find the total valence electrons in a if5 (iodine pentafluoride) molecule, first of all you should know the valence electrons present in iodine atom as well as fluorine atom. These are then utilized to create water and oil repellent emulsions for the treatment of textiles and for fire extinguishing froths. Let us focus on the shape of if 5 and also some important points in a different segment. We need to draw the lewis dot structure of hydrogen. Iodine (i) is the central atom as it is less electronegative than fluorine. It will hold more than 8 electrons. Draw the lewis structure for the iodide pentafluoride molecule. If 5 is the molecular formula of iodine pentafluoride. I does not follow the octet rule. It is one of the fluorides of iodine. Web solution for draw the lewis structure for the iodide pentafluoride (ifs) molecule. It is a colorless liquid. Web we begin our discussion of the relationship between structure and bonding in covalent compounds by describing the interaction between two identical neutral atoms—for example, the h 2 molecule, which contains a purely covalent bond. If 5 is the molecular formula of. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. This widget gets the lewis structure of chemical compounds. We need to draw the lewis dot structure of hydrogen. Web draw a plausible lewis dot structure for the iodine pentafluoride (if 5) molecule. In order to find the total valence electrons in a. Steps of drawing if5 lewis structure. Iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it. Web draw a plausible lewis dot structure for the iodine pentafluoride (if 5) molecule. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Figure out. Find the total valence electrons in if5 molecule. If 5 is the molecular formula of iodine pentafluoride. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw a plausible lewis dot structure for the iodine pentafluoride (if. Web iodine pentafluoride is an interhalogen compound with chemical formula if 5. Write the correct skeletal structure for the molecule. In short, these are the steps you need to follow for drawing a lewis structure: Web first, we need to draw the lewis structure of if5. 1 is the elected configuration of hydrogen. Draw the lewis structure for the iodide pentafluoride molecule. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web draw a plausible lewis dot structure for the iodine pentafluoride (if 5) molecule. In short, these are the steps you need to follow for drawing a lewis structure: Web solution for draw the lewis structure. In the lewis structure of if5, there are five fluorines connected with a single bond surrounding the central atom i. Web steps to draw the lewis structure: Web first, we need to draw the lewis structure of if5. 7 valence electrons (group 17) five fluorine (f) atoms: It is one of the fluorides of iodine. Web july 11, 2022 by arpita yadav. 5 × 7 valence electrons = 35 valence electrons. It is one of the fluorides of iodine. Iodine pentafluoride (if5) has 5 fluorine atoms bonded to a central iodine, and that iodine has one lone pair on it. Count the total valence electrons: This problem has been solved! These are then utilized to create water and oil repellent emulsions for the treatment of textiles and for fire extinguishing froths. Web draw a plausible lewis dot structure for the iodine pentafluoride (if 5) molecule. Steps of drawing if5 lewis structure. Let us focus on the shape of if 5 and also some important points. Iodine is below period two on the periodic table so it can have an expanded octet (hold more than. It is a colorless liquid, although impure samples appear yellow. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web steps to draw the lewis structure: Let us focus on the shape of if. It will hold more than 8 electrons. These are then utilized to create water and oil repellent emulsions for the treatment of textiles and for fire extinguishing froths. Let us focus on the shape of if 5 and also some important points in a different segment. Iodine having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. He bromine pentafluoride (brf5) molecule.draw the lewis structure for the bromine pentafluoride (brf5) molecule. Web first, we need to draw the lewis structure of if5. 7 + 35 = 42 electrons. 11k views 4 years ago lewis structures. Find more chemistry widgets in wolfram|alpha. In short, these are the steps you need to follow for drawing a lewis structure: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Iodine (i) is the central atom as it is less electronegative than fluorine. It is used as a fluorination reagent and even a solvent in specialized syntheses. Figure out how many electrons the molecule must have, based on the number of valence electrons in each. This gives it ax5e1 shape by. Draw the lewis structure for the iodide pentafluoride molecule.
SOLVED Draw the Lewis structure for the iodide pentafluoride (IFs

Iodine pentafluoride IF5 Molecular Geometry Hybridization
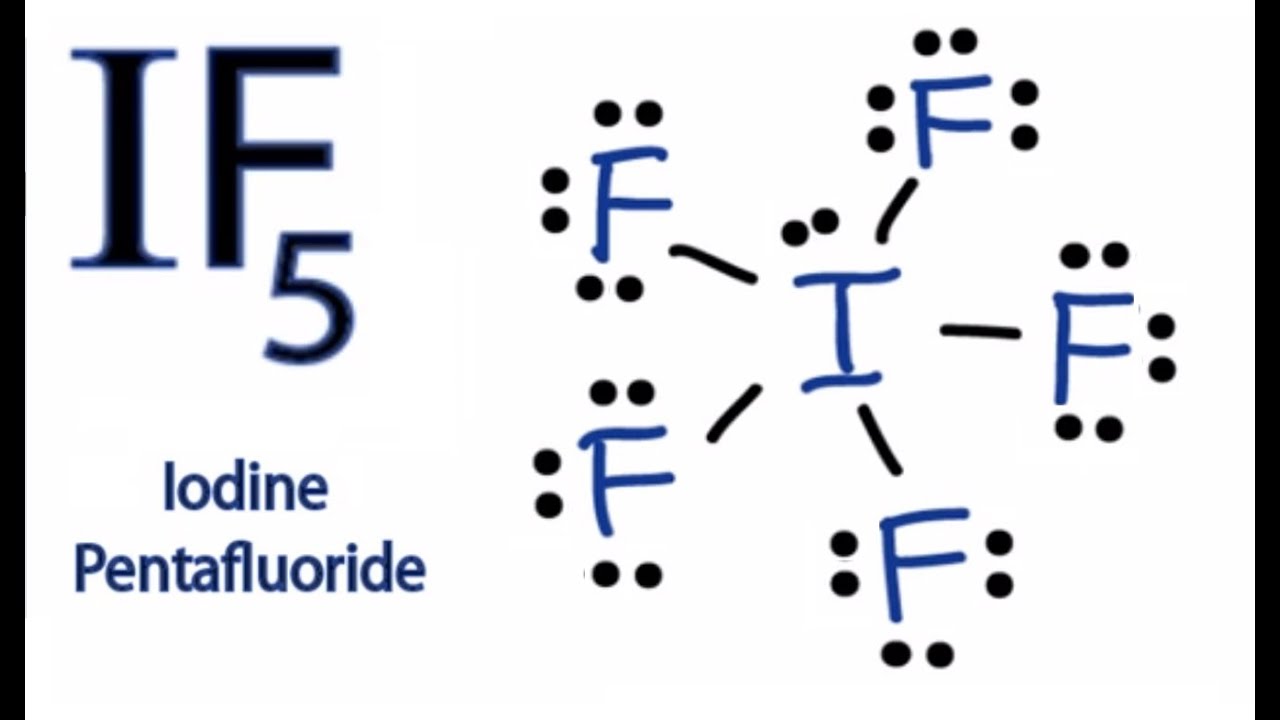
Iodine Pentafluoride Lewis Structure

IF5 Lewis Structure (Iodine Pentafluoride) YouTube
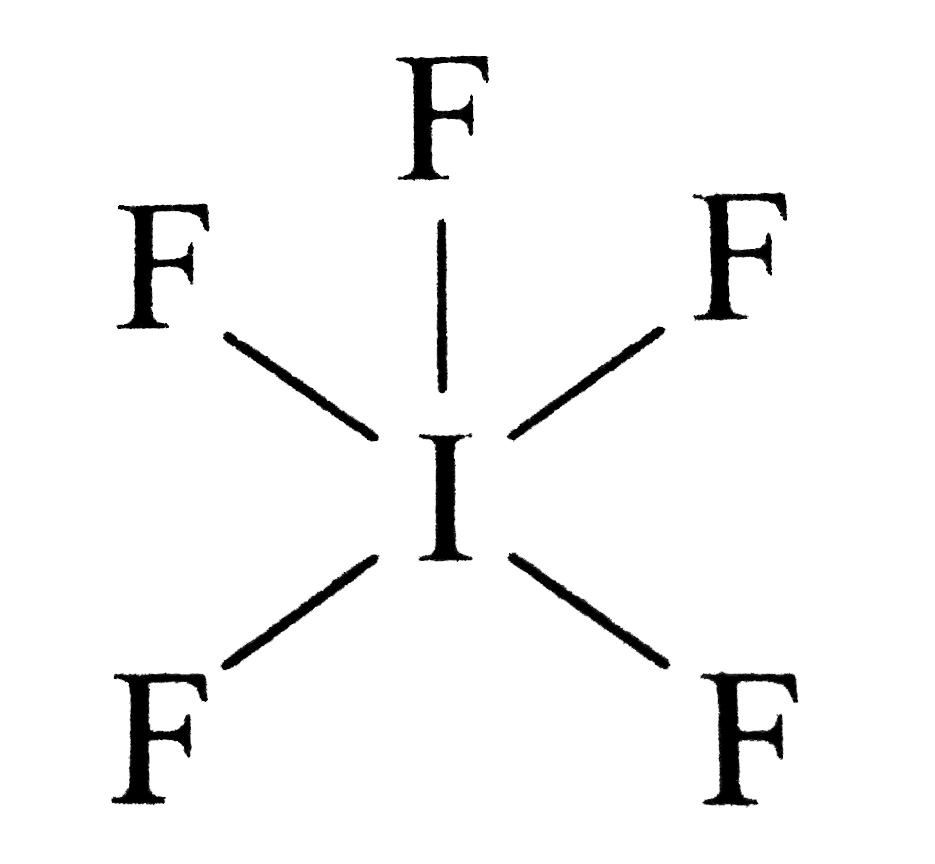
Draw the Lewis structure of iodine pentafluoride, IF5.

Iodine Pentafluoride Lewis Structure

SOLVED Draw the Lewis structure for the iodide pentafluoride (TFs

Draw The Lewis Structure Of If5 Iodine Pentafluoride Youtube Otosection
Solved Draw the Lewis structure for the iodide pentafluoride

Iodine Pentafluoride Lewis Structure
1 Is The Elected Configuration Of Hydrogen.
The Following Procedure Will Give You The Correct Lewis Structure For Any Molecule Or Polyatomic Ion That Has One Central Atom.
This Widget Gets The Lewis Structure Of Chemical Compounds.
Iodine Is Below Period Two On The Periodic Table So It Can Have An Expanded Octet (Hold More Than.
Related Post: