Draw The Lewis Structure For The Hcn
Draw The Lewis Structure For The Hcn - Web subtract step 1 total from step 2. Web chemistry learning made easy.this tutorial will help you deal with the lewis structure and moleculargeometry for hydrogen cyanide (hcn). Subtract step 3 number from step 1. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable. Now, use the information from step 4 and 5 to draw the lewis. Before we can start drawing the lewis structure, we need to determine the total number of valence electrons in hcn. Determine the total number of valence (outer shell) electrons. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer atoms (except h) to complete their octets. Web hey guys!in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Include all lone pairs of electrons. (valence electrons are the number of electrons present in the outermost shell of an atom). Draw the lewis structure for the hcn molecule. (there are molecules, like o 2, which have unpaired electrons even though they could all be paired, but you. Web subtract step 1 total from step 2. Determine the total number of valence (outer shell) electrons. All electrons should be in lone pairs or bonding pairs. Make sure you put the correct atom at the center of the hcn molecule. When you draw the lewis structure, make all the electrons paired unless there is an odd number of electrons. Draw the lewis structure for hcn. In order to draw the lewis structure of hcn, first of. All electrons should be in lone pairs or bonding pairs. Web the hcn molecule consists of three atoms: This is crucial because valence electrons are the ones involved in chemical bonding. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of. Web subtract step 1 total from step 2. The hydrogen atom is bonded to the carbon atom, and the nitrogen atom is bonded to the carbon atom via a triple bond. Simplified lewis structure drawing for nonscience majors. journal of chemical education 75 (1998): Web to draw the lewis dot structure of any molecule, it is essential to know the. Web drawing the lewis structure for hcn. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. Some compounds have a very unique and different lewis structure and hcn is one of those. The lewis structure of hcn shows that the carbon atom is the central atom and is. Web the lewis structure (lewis dot diagram) for hcn.1. Calculate the total number of valence electrons. Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. Make sure you put the correct atom. The molecule is made up of one hydrogen ato. Web the hcn molecule consists of three atoms: The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms. It can be found. Determine the total number of valence (outer shell) electrons. Hydrogen (h), carbon (c), and nitrogen (n). The sum of the valence electrons is 5 (from n) + 6 (from o) = 11. Web lewis structure of hcn. Some compounds have a very unique and different lewis structure and hcn is one of those. Determine the total number of valence (outer shell) electrons. Now we will draw the lewis dot structure of the compound. Subtract step 3 number from step 1. When you draw the lewis structure, make all the electrons paired unless there is an odd number of electrons. (there are molecules, like o 2, which have unpaired electrons even though they could. With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms. Web hey guys!in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Some compounds have a very unique and different lewis structure and hcn is one of those.. Draw the lewis structure for the hcn molecule. All electrons should be in lone pairs or bonding pairs. With the lewis structure for hcn you’ll need to share more than one pair of electrons between the carbon and the nitrogen atoms. Web subtract step 1 total from step 2. Web draw out a correct lewis structure for the following compounds. Hcn consists of three atoms: This is crucial because valence electrons are the ones involved in chemical bonding. Draw the lewis structure for hcn. Subtract step 3 number from step 1. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if required #4 convert lone pairs of the atoms, and minimize formal charges #5 repeat step 4 if needed, until all charges are minimized, to get a stable. Web lewis structure of hcn. The final answer must have this number of electrons‼! The hydrogen atom is bonded to the carbon atom, and the nitrogen atom is bonded to the carbon atom via a triple bond. (1 × × 1) + (4 × × 1. Hydrogen (h), carbon (c), and nitrogen (n). (valence electrons are the number of electrons present in the outermost shell of an atom).
How do you draw the Lewis structure of HCN (hydrogen cyanide)? HCN

Hcn Lewis Structure Bonds Draw Easy
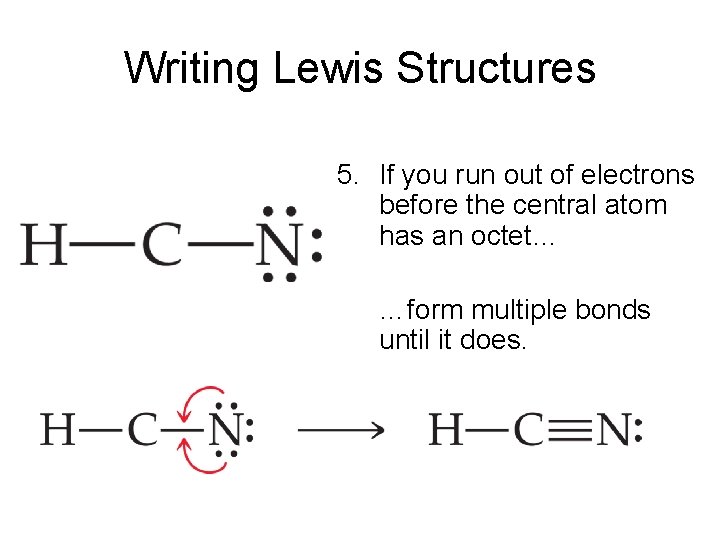
Hcn Lewis Structure Bonds Draw Easy

Lewis Diagram For Hcn

HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
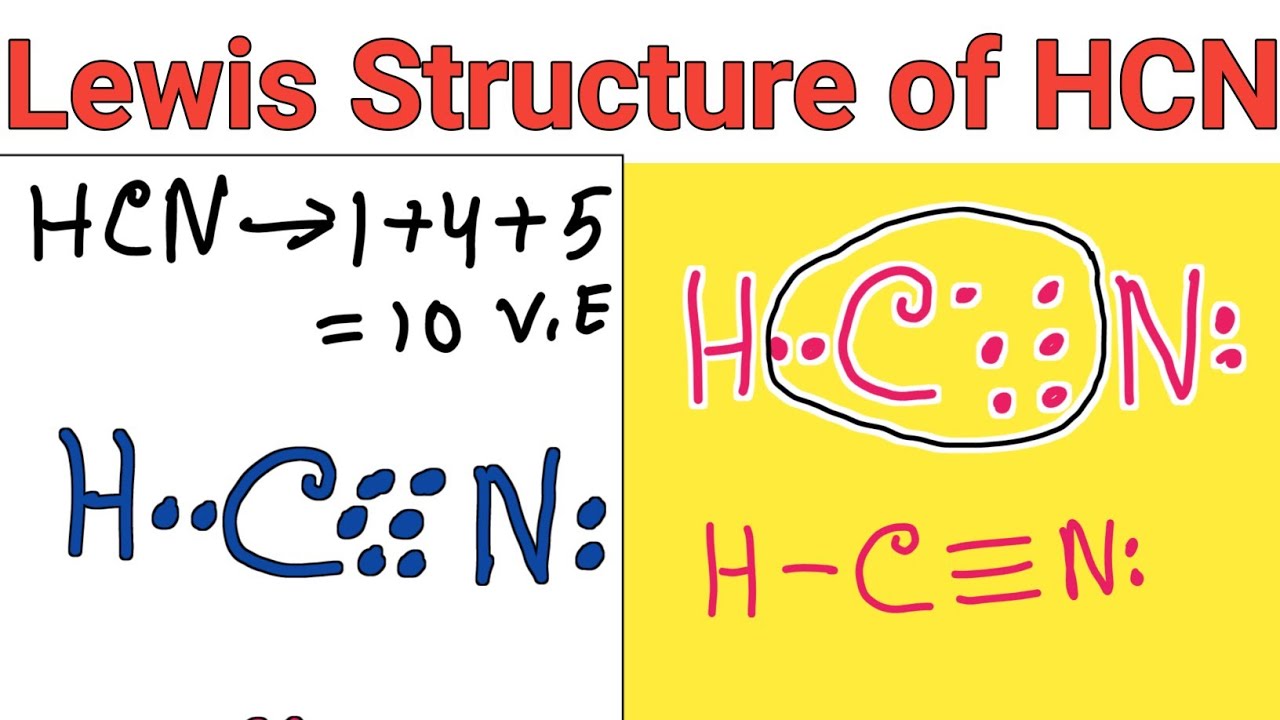
Lewis structure of HCN (Hydrogen cyanide) YouTube
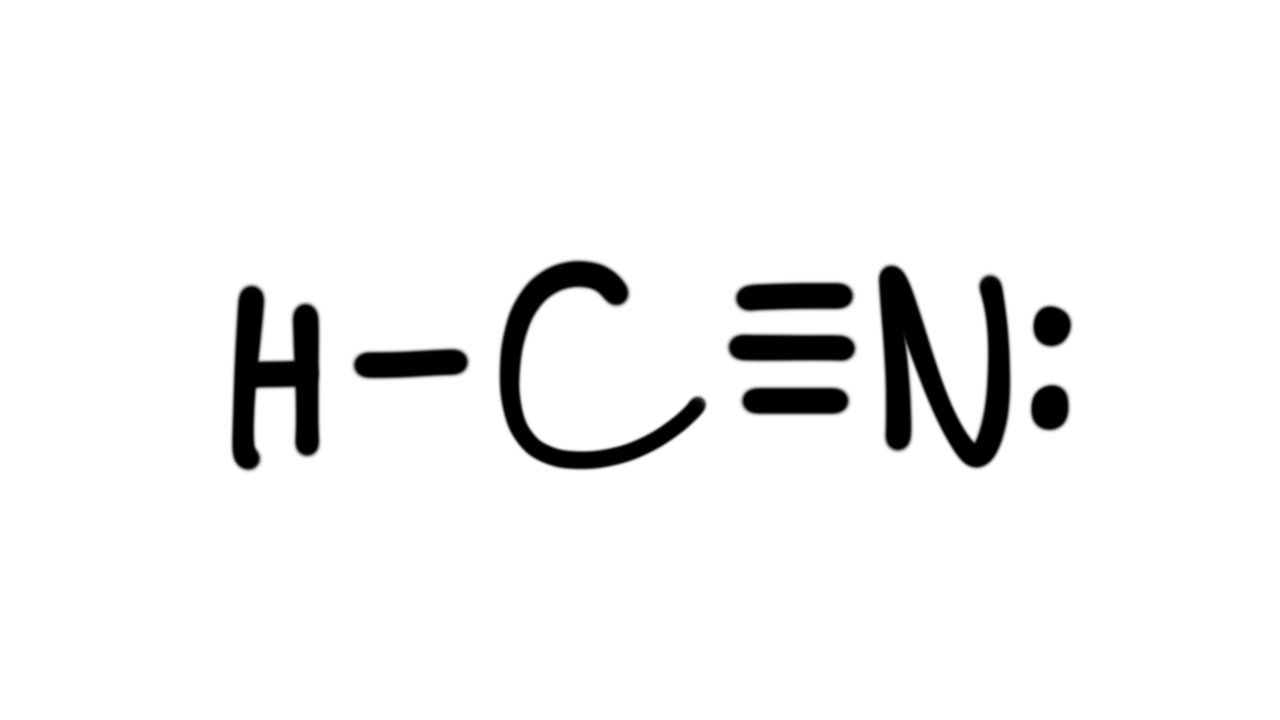
Lewis Diagram For Hcn

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and

HCN Lewis Structure (Hydrogen Cyanide) YouTube
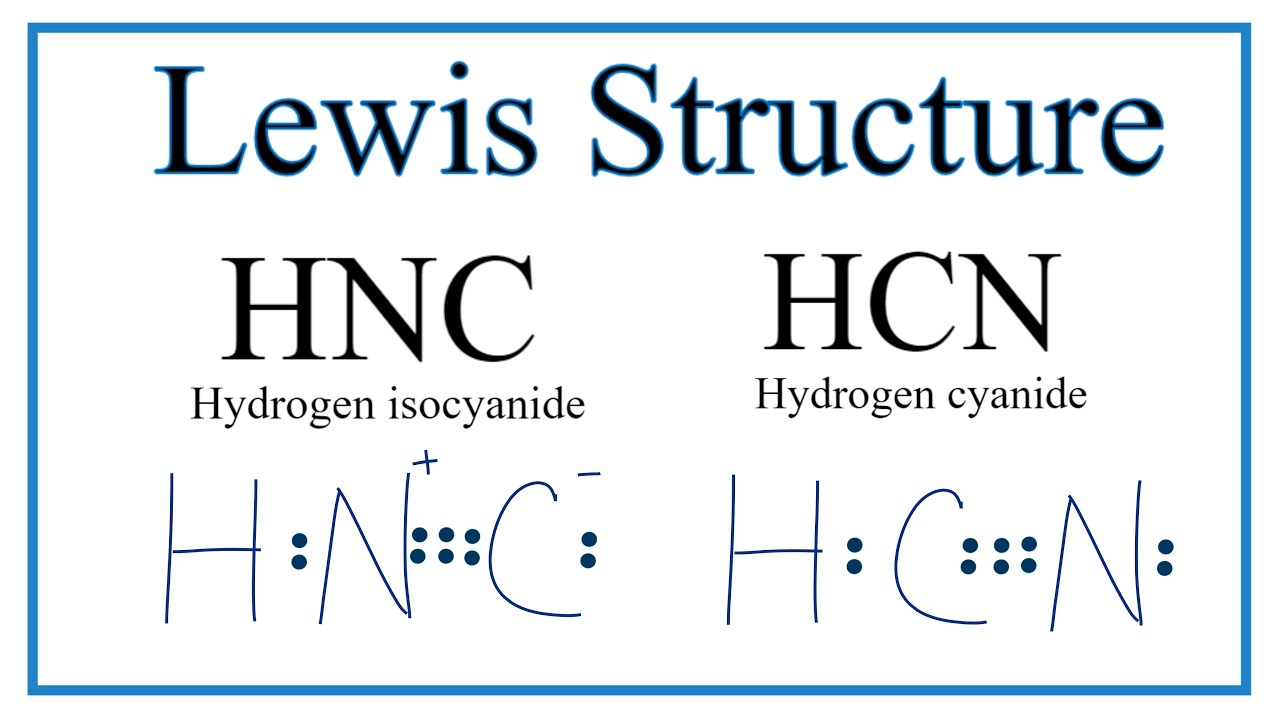
Hcn Lewis Structure Bonds Draw Easy
It Can Be Found In Fruits That Have Pits Due To The Fact That.
The Lewis Structure Of Hcn Shows That The Carbon Atom Is The Central Atom And Is Covalently Bonded To Both Hydrogen And Nitrogen Atoms.
Watch This Video And Learn The Steps And Rules For Drawing The Lewis Dot Diagram Of Hcn, A Polar.
Web How To Draw The Lewis Dot Structure Of Hydrogen Cyanide (Hcn) Molecule In A Simple And Easy Way?
Related Post: