Draw The Lewis Structure For The Bromine Difluoride Ion
Draw The Lewis Structure For The Bromine Difluoride Ion - (valence electrons are the number of electrons present in the outermost shell of an atom). Web every chemistry student has to learn how to draw lewis dot structures. Many of the ions that form have eight electrons in their valence shell. Web drawing lewis structures for molecules with one central atom: This means that the central bromine (br) atom will have an odd number (9 total). This problem has been solved! Web the hybridization of the atoms in this idealized lewis structure is given in the table below. The lewis structure for brf is similar to other structures like clf or f 2. With brf 2 there are an odd number of valence electrons (21 total). The bromine atom (br) is at the center and it is surrounded by 4 fluorine atoms (f). Bromine is the least electronegative and goes at the center of the brf 2. Here, the given molecule is brf. This problem has been solved! Web the hybridization of the atoms in this idealized lewis structure is given in the table below. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has. Web every chemistry student has to learn how to draw lewis dot structures. Bromine is the least electronegative and goes at the center of the brf 2. If the molecule exhibits resonance, draw only one (1) of the resonance forms. Please note that your structure can't be well described by a single lewis structure, because of extensive delocalization. The lewis. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Many of the ions that form have eight electrons in their valence shell. (valence electrons are the number of electrons present in the outermost shell of an atom). There is a single bond between the bromine atom (br) and each fluorine atom (f). Web. If the molecule exhibits resonance, draw only one (1) of the resonance forms. Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one. In order to draw the lewis structure of brf, first of all you have to find the total number of valence electrons present in the brf molecule. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). The bromine atom (br) is at the center and it. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). Figure out how many electrons the molecule must have, based on the number of valence electrons in each. Assess the stability of a structure by considering formal charges of atoms. You'll get a detailed solution from a. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). How the molecule might react with other molecules. Give examples for molecules and ions that do not follow the octet rule. Figure out how many electrons the molecule must have, based on the number of valence electrons. With brf lewis structure there are a total of 14 valence electrons. Web drawing lewis structures for molecules with one central atom: Web every chemistry student has to learn how to draw lewis dot structures. Calculate the total number of valence electrons. Assess the stability of a structure by considering formal charges of atoms. Give examples for molecules and ions that do not follow the octet rule. Many of the ions that form have eight electrons in their valence shell. Web drawing the lewis structure for brf. Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double. How the molecule might react with other molecules. Determine the total number of valence electrons in the molecule or ion. The key is to understand the steps and practice. Web the following procedure can be used to draw lewis structure for simple molecules. Figure out how many electrons the molecule must have, based on the number of valence electrons in. There is a single bond between the bromine atom (br) and each fluorine atom (f). Calculate the total number of valence electrons. This means that the central bromine (br) atom will have an odd number (9 total). The astute reader may have noticed something: The key is to understand the steps and practice. Web draw the lewis structure for the bromine difluoride (brf2) ion. Web the following procedure can be used to draw lewis structure for simple molecules. Web drawing the lewis structure for brf 2. Calculate the total number of valence electrons. Many of the ions that form have eight electrons in their valence shell. How the molecule might react with other molecules. (b) which form of dichloroethylene has a dipole moment of 2.39 d, and which has. Assess the stability of a structure by considering formal charges of atoms. Web this widget gets the lewis structure of chemical compounds. In order to draw the lewis structure of brf, first of all you have to find the total number of valence electrons present in the brf molecule. Web drawing the lewis structure for brf.
Lewis Dot Diagram For Bromine Diagram For You
Lewis structure Bromine pentafluoride Sulfur tetrafluoride Xenon
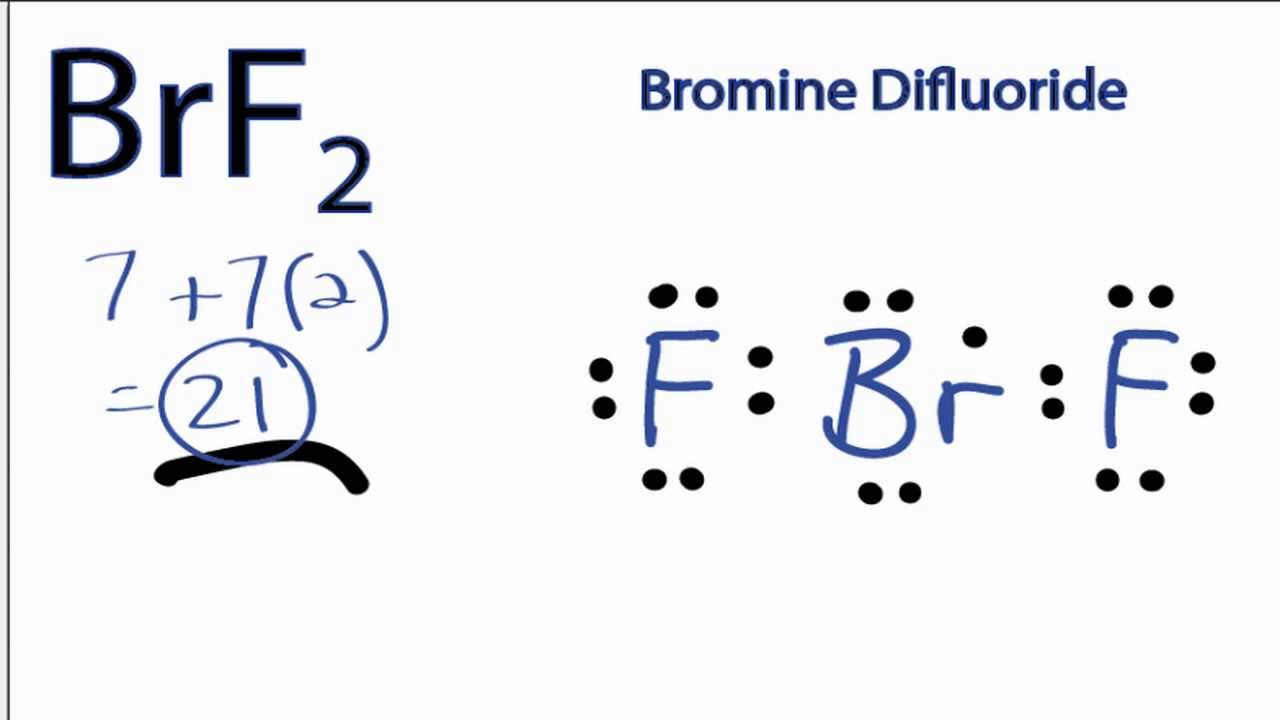
BrF2 Lewis Structure How to Draw the Lewis Structure for Bromine

Bromine Lewis Dot Structure Drawing, Several Compounds and Detailed

How to Draw the Lewis Dot Structure for BrF2 YouTube
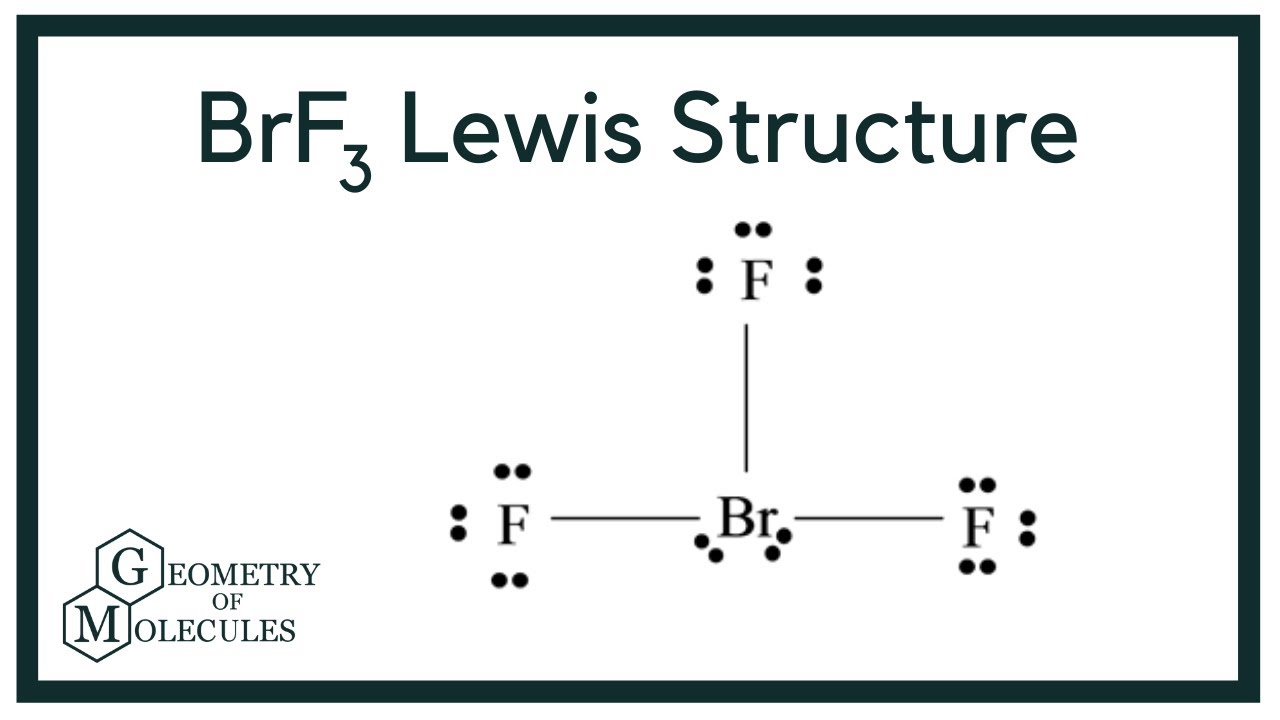
BrF3 Lewis Structure (Bromine Trifluoride) YouTube

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
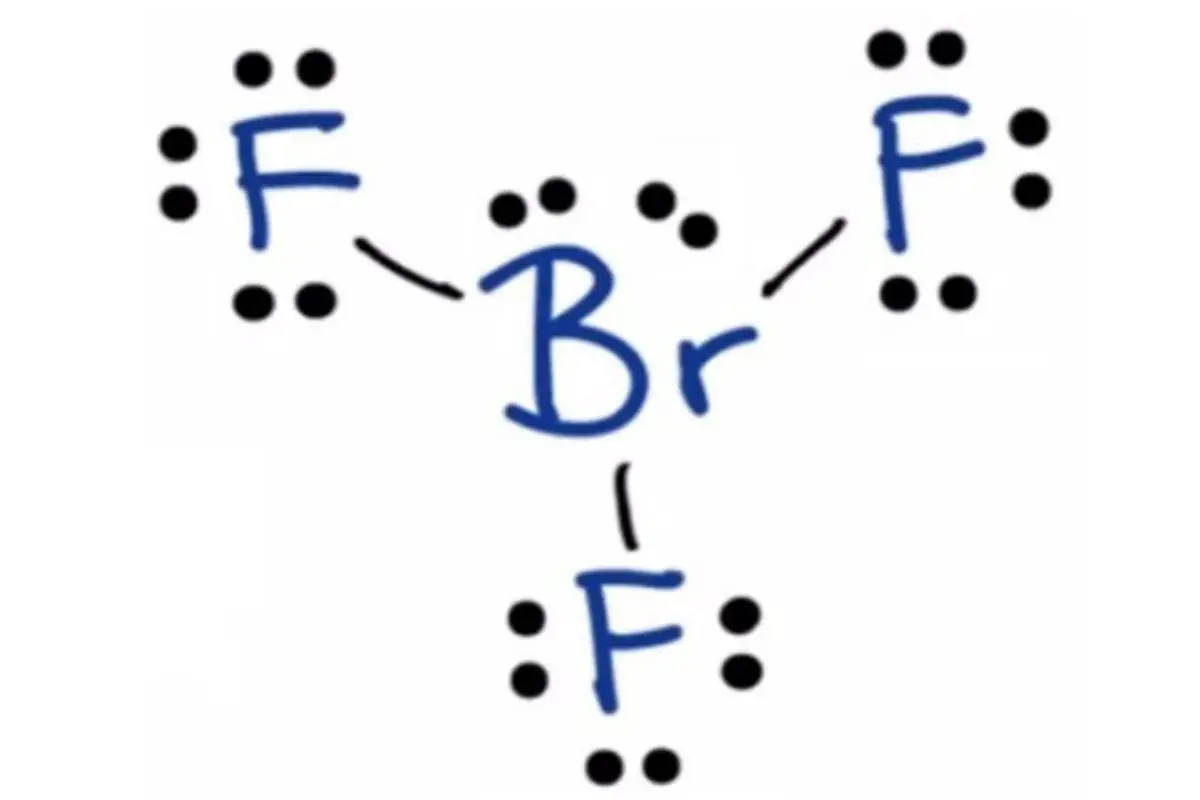
BrF3 Molecular Geometry (2021) Everything You Need to Know

DOWNLOAD How To Draw The Lewis Dot Structure For Brf5 Bromine
Solved Draw the Lewis structure of the bromine difluoride
Please Note That Your Structure Can't Be Well Described By A Single Lewis Structure, Because Of Extensive Delocalization.
In Section 4.7, We Demonstrated That Ions Are Formed By Losing Electrons To Make Cations, Or By Gaining Electrons To Form Anions.
With Brf Lewis Structure There Are A Total Of 14 Valence Electrons.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Related Post: