Draw The Lewis Structure For Cocl2
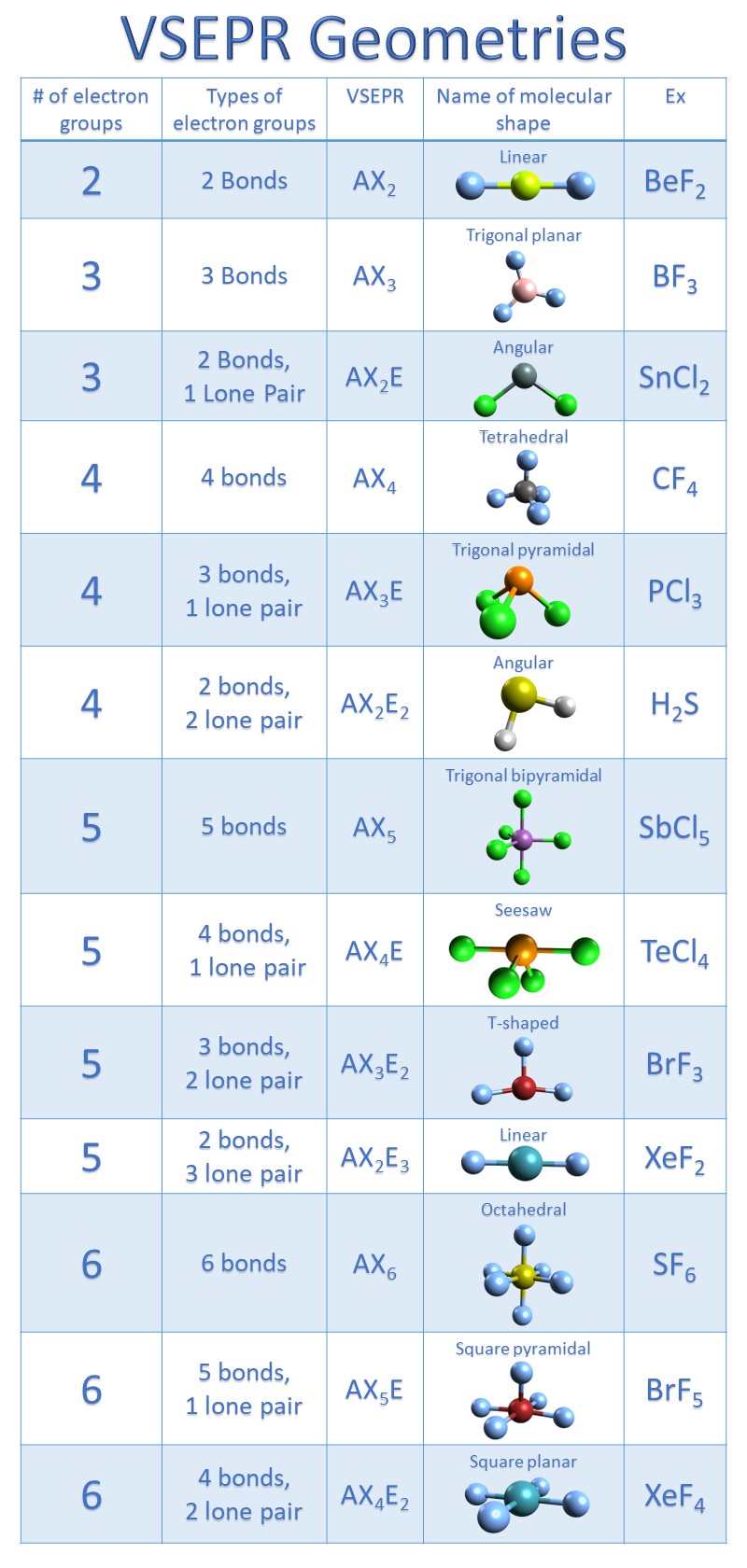
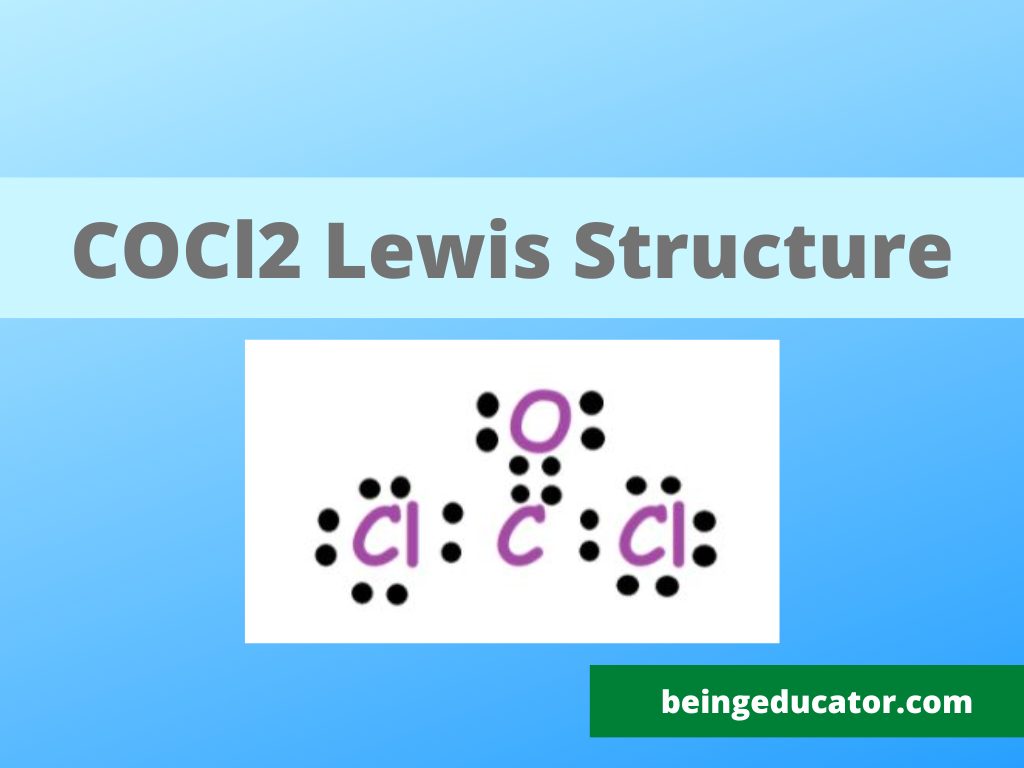
Draw The Lewis Structure For Cocl2 - 4 + (3 × 6) + 2 = 24 electrons. One uses math, the other puzzle pieces to give the three correct structure. Because carbon is less electronegative than oxygen and hydrogen is normally terminal, c must be the central atom. We follow the following steps to draw the lewis structure of the molecule. Draw the lewis structure for cocl2, including lone pairs. Bent linear tetrahedral trigonal planar trigonal pyramidal what is the cl−c−cl bood angle?what is the molecular shape of cocl2 ? It is used widely to make plastics and pesticides. Calculate the total valence electrons in the molecule. 1×6 = 6 total = 24 valence electrons. Web steps of drawing cocl2 lewis structure step 1: Web cocl2 is a chemical compound, known by the name ‘phosgene’. You'll need to form a double bond. Find more chemistry widgets in wolfram|alpha. Web 6 steps to draw the lewis structure of cocl2 step #1: For cocl 2, it is as shown below: Because carbon is less electronegative than oxygen and hydrogen is normally terminal, c must be the central atom. 1×4 = 4 cl : Web steps of drawing cocl2 lewis structure step 1: Web cocl2 is a chemical compound, known by the name ‘phosgene’. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and. The final answer must have this number of electrons‼! Count the total number of valence electrons first, we need to determine the total number of valence electrons available in the molecule. Web steps of drawing cocl2 lewis structure step 1: Because carbon is less electronegative than oxygen and hydrogen is normally terminal, c must be the central atom. It has. You'll need to form a double bond. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Calculate the total valence electrons in the molecule. View the full answer step 2. Web hello guys!phosgene having a chemical formula of cocl2 is a polyatomic molecule. Calculate the total valence electrons in the molecule. Web drawing the lewis structure for cocl 2. Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. 8 + (6 × 7) = 50. Web hello guys!phosgene having a chemical formula of cocl2 is a polyatomic molecule. Web steps of drawing cocl2 lewis structure step 1: Bent linear tetrahedral trigonal planar trigonal pyramidal what is the cl−c−cl bond angle? Here, the given molecule is cocl2. Let's break down the process of drawing the lewis structure for cocl2 (phosgene) into clear steps. What is the molecular shape of cocl2 ? Web cocl2 is a chemical compound, known by the name ‘phosgene’. Web the ch2o c h 2 o molecule. Web drawing the lewis structure for cocl 2. The lewis electron structure is drawn within brackets as is customary for a molecular ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a. In the cocl 2 lewis structure, carbon is less electron electronegative than oxygen and goes in the center of the lewis structure (note that hydrogen atoms always go on the outside).; Draw the lewis structure for cocl2, including lone pairs. Find the total valence electrons in cocl2 molecule. 1×6 = 6 total = 24 valence electrons. Find more chemistry widgets. Include all lone pairs of electrons. Web this problem has been solved! 1×4 = 4 cl : Carbon (c) has 4 valence electrons, oxygen (o) has 6, and each chlorine (cl) atom has 7. We follow the following steps to draw the lewis structure of the molecule. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 98.92 gram/mol. Web we show two methods to find correct lewis structure of cocl2. 8 + (2 × 7) = 22. Draw the lewis structure of phosgene, cocl2. View the full answer step 2. Therefore, the lewis structure for the cocl 2 is represented as follows:. Calculate the total number of valence electrons. Web hello guys!phosgene having a chemical formula of cocl2 is a polyatomic molecule. View the full answer step 2. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence. Count the total number of valence electrons first, we need to determine the total number of valence electrons available in the molecule. Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Find the total valence electrons in cocl2 molecule. Web to properly draw the cocl 2 lewis structure, follow these steps: Bent linear tetrahedral trigonal planar trigonal pyramidal what is the cl−c−cl bood angle?what is the molecular shape of cocl2 ? In the lewis structure for cocl 2 there are a total of 24 valence electrons.; Web steps of drawing cocl2 lewis structure step 1: (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs (sp). A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. What is the molecular shape of cocl2 ?
How to Calculate the Formal Charge of CoCl2 Sciencing

Cocl2 Lewis Dot Structure Draw Easy
![The Lewis Structure of COCl2 [with free study guide and video]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/COCl2-lewis-structure.jpg)
The Lewis Structure of COCl2 [with free study guide and video]

Cocl2 Lewis Structure Shape Draw Easy

COCl2 Lewis Structure (Phosgene) YouTube
Lewis Structure Of Cocl2
COCl2 Lewis Structure Hybridization Polarity Molecular Geometry

Lewis Dot Structure For Cocl2
SOLVED Draw the Lewis structure for COCl2, including lone pairs.

COCl2 Lewis Structure How to Draw the Lewis Structure for COCl2 YouTube
In The Cocl 2 Lewis Structure, Carbon Is Less Electron Electronegative Than Oxygen And Goes In The Center Of The Lewis Structure (Note That Hydrogen Atoms Always Go On The Outside).;
Web The Formal Charges Being 0 For All Of The Atoms In The Cocl 2 Molecule Tells Us That The Lewis Dot Structure Presented Above Is Stable.
Step 2) Attach The Atoms To Each Other Using Single Bonds (“Draw The Skeleton Structure”) Step 3) Add Electrons To All Outer Atoms (Except H) To Complete Their Octets.
We Follow The Following Steps To Draw The Lewis Structure Of The Molecule.
Related Post: