Draw The Lewis Structure For A Nitric Oxide Ion
Draw The Lewis Structure For A Nitric Oxide Ion - To draw the lewis structure for a nitric oxide (no) ion, firstly identify the total number of valence electrons by adding up the valence electrons of nitrogen (n), oxygen (o), and an additional electron for the negative charge. Draw lewis structures for the molecule n_2h_4. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. 4 valence electrons/atom×1 atom = 4 + h: Draw the lewis structure for a nitric oxide no− ion. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. Web answer to solved draw the lewis structure for a nitric oxide (no) ion. Draw the lewis structure for a nitric oxide (no) ion. Draw a lewis structure for each ion. Nitrogen oxide, ion permanent link for this species. If you need more information about formal charges, see lewis structures. For a molecule, we add the number of valence electrons on each atom in the molecule: Draw lewis structures for the molecule n_2h_4. Web let us determine the lewis structures of sih 4, cho 2 −, no +, and of 2 as examples in following this procedure: If we. 4 valence electrons/atom×1 atom = 4 + h: Web here’s how to approach this question. Draw lewis structures for the molecule n_2h_4. Determine the total number of valence (outer shell) electrons in the molecule or ion. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. 4 valence electrons/atom×1 atom = 4 + h: Nitric oxide has 11 valence electrons. Web here’s how to approach this question. For a molecule, we add the number of valence electrons on each atom in the molecule: Draw a lewis structure for the following. It is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Determine the total number of valence (outer shell) electrons in the molecule or ion. There’s just one step. Web here’s how to approach this question. Draw a lewis structure for each ion. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. There’s just one step to solve this. For a molecule, we add the number of valence electrons on each atom in the molecule: This structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Web no (nitric oxide) is an oxide of nitrogen. It's lewis structure can be drawn by following vsepr rule. Draw a lewis structure for each ion. 4 valence electrons/atom×1 atom = 4 +. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web here’s how to approach this question. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. It's. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. Connect the atoms to each other with single bonds to form a “skeleton structure.”. Draw the lewis structure for a nitric oxide no− ion. Nitric oxide has 11 valence electrons. Draw the lewis structure for a nitric oxide no− ion. Draw the lewis structure for a nitric oxide (no) ion. Web here’s how to approach this question. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. If we were to imagine nitric oxide had ten valence electrons we would come up with the lewis structure (figure \(\pageindex{1}\)): If you need more. Determine the total number of valence (outer shell) electrons in the molecule or ion. We can draw the lewis structure of any covalent molecule by following the six steps. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. Get the free lewis structure finder widget for your website, blog, wordpress, blogger,. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. Draw a lewis structure for the following. It is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. It's lewis structure can be drawn by following vsepr rule. Web let us determine the lewis structures of sih 4, cho 2 −, no +, and of 2 as examples in following this procedure: Draw the lewis structure for a nitric oxide no− ion. Use this link for bookmarking this species for. It is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. Web this widget gets the lewis structure of chemical compounds. Web an example of a stable molecule with an odd number of valence electrons would be nitric oxide. One way this species is produced is in internal combustion engines when oxygen and nitrogen react at high temperatures. Draw the lewis structure for a nitric oxide (no) ion. If you need more information about formal charges, see lewis structures. Web nitric oxide, no, which is produced in internal combustion engines when oxygen and nitrogen react at high temperatures, is an example.
NO Lewis Structure How to Draw the Lewis Structure for NO (Nitric

Structures of Oxides of Nitrogen Trick for Lewis Dot Structures p

NO Lewis Structure How To Draw The Lewis Structure For NO(Nitric
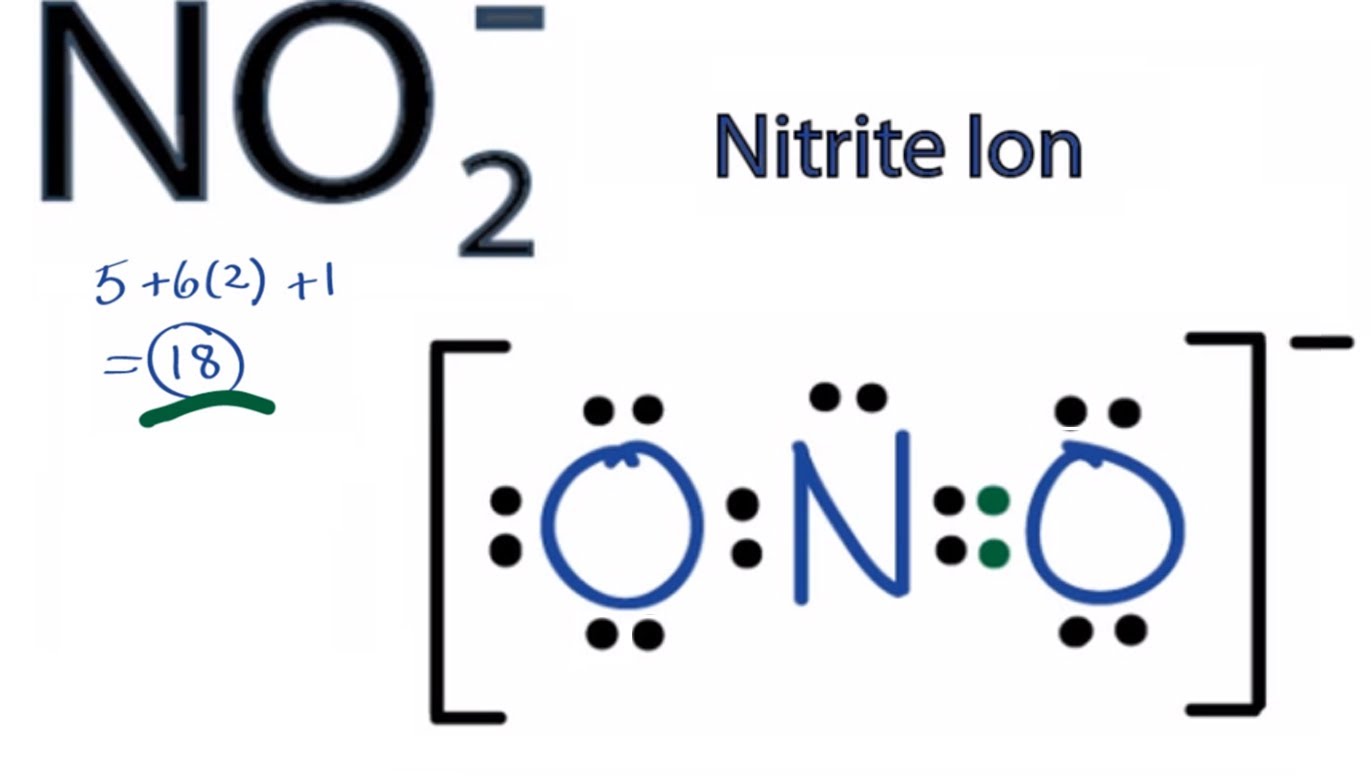
Drawing Lewis Structures Chemistry Socratic

Nitric oxide molecule skeletal formula Royalty Free Vector
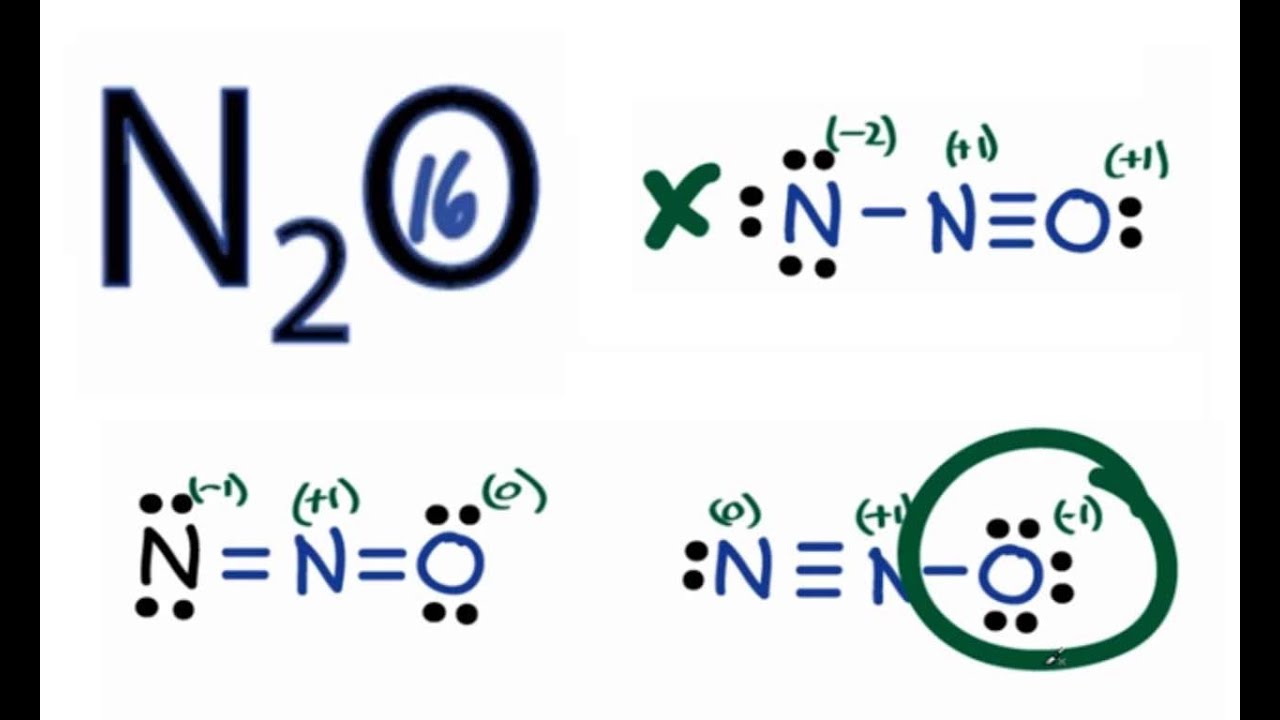
N2O Lewis Structure How to Draw the Lewis Structure for N2O YouTube

Nitric Oxide Molecular Orbital Diagram

Nitric oxide molecule skeletal formula Royalty Free Vector
![]()
[Solved] Lewis structure of nitric oxide 9to5Science
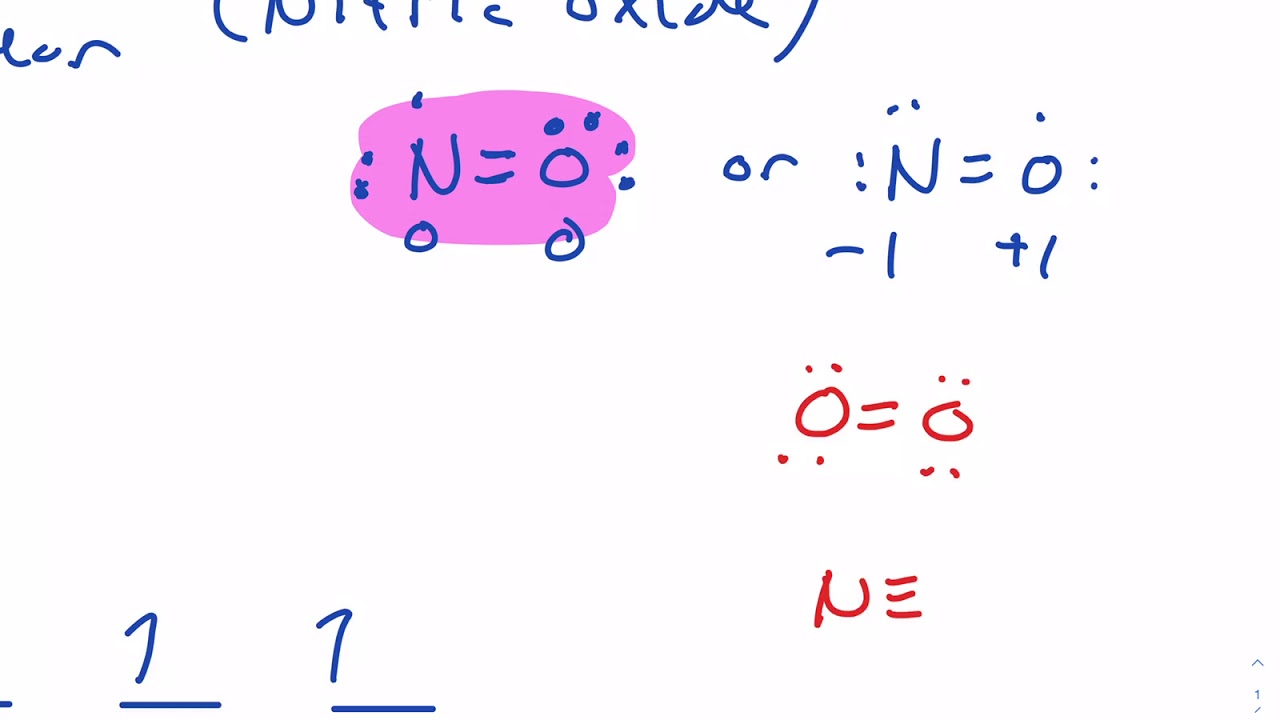
Molecular Orbital Diagram for Nitric Oxide Heteronuclear molecule YouTube
Draw Lewis Structures For The Molecule N_2H_4.
Web No (Nitric Oxide) Is An Oxide Of Nitrogen.
Number Of Electrons In The Valence Shells Are Used To Draw The Stable Lewis Structure And It Is Used Find The Resonance Structures Of No.
If We Were To Imagine Nitric Oxide Had Ten Valence Electrons We Would Come Up With The Lewis Structure (Figure \(\Pageindex{1}\)):
Related Post: