Draw The Electron Configuration For A Neutral Atom Of Sulfur
Draw The Electron Configuration For A Neutral Atom Of Sulfur - The aufbau principle states that electrons are always placed in the. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around. Web intro to electron configurations; This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p. Using only the periodic table; Make certain that you can define, and use in context, the key terms below. Web the electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. When applying this pattern, two rules must be followed: Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Web to write electron configurations and draw orbital box diagrams, there are three rules that must be applied. When applying this pattern, two rules must be followed: Electron configuration of oxygen (o) [he] 2s 2 2p 4: This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p. In order to maximize their stability, electrons will always prefer to occupy the lowest energy orbital. 1s 2 2s 2 2p. The electron configuration for a neutral sulfur atom seems to. 1s 2 2s 2 2p 3: Web this raises an interesting question: Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: Electron configuration of oxygen (o) [he] 2s 2 2p 4: When applying this pattern, two rules must be followed: How does the sulfur atom in sf 4 hold 10 electrons in its valence shell? Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. Web this raises an interesting question: In this article, we will study how. Web to write electron configurations and draw orbital box diagrams, there are three rules that must be applied. The electron configuration for a neutral sulfur atom seems to. Otherwise, write the order of the. Using only the periodic table; Web what is the electron configuration of: The electron configuration for a sulfur atom is [ne]3s23p4 and its lewis symbol is shown in the figure below. How does the sulfur atom in sf 4 hold 10 electrons in its valence shell? Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. In order to. The electron configuration for a neutral sulfur atom seems to. In this article, we will study how electrons are arranged in different shells and subshells of sulphur. Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o). Otherwise, write the order of the. When applying this pattern, two rules must be followed: Using only the periodic table; Web to write electron configurations and draw orbital box diagrams, there are three rules that must be applied. 1s 2 2s 2 2p 4: Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots. Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: 1s 2 2s 2 2p 4: Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence. Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web the electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. Otherwise, write the order of the. 1s2 2s2 2p6 3s2 3p4. Web the electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o). Web about press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features nfl sunday ticket. 1s 2 2s 2 2p 4: Web find the. Web electron configuration of nitrogen (n) [he] 2s 2 2p 3: Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Make certain that you can define, and use in context, the key terms below. This means that sulfur has two electrons in its 1s orbital, two in its 2s orbital, six in its 2p. Otherwise, write the order of the. 1s2 2s2 2p6 3s2 3p4. Web to write electron configurations and draw orbital box diagrams, there are three rules that must be applied. Web a lewis electron dot symbol (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around. O electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. Web the electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. 1s 2 2s 2 2p 4: Web sulphur electron configuration is: 1s 2 2s 2 2p 3: The electron configuration for a neutral sulfur atom seems to. Web the electron configuration of sulfur is 1s^2 2s^2 2p^6 3s^2 3p^4. How does the sulfur atom in sf 4 hold 10 electrons in its valence shell?
Sulfur Bohr Model — Diagram, Steps to Draw Techiescientist
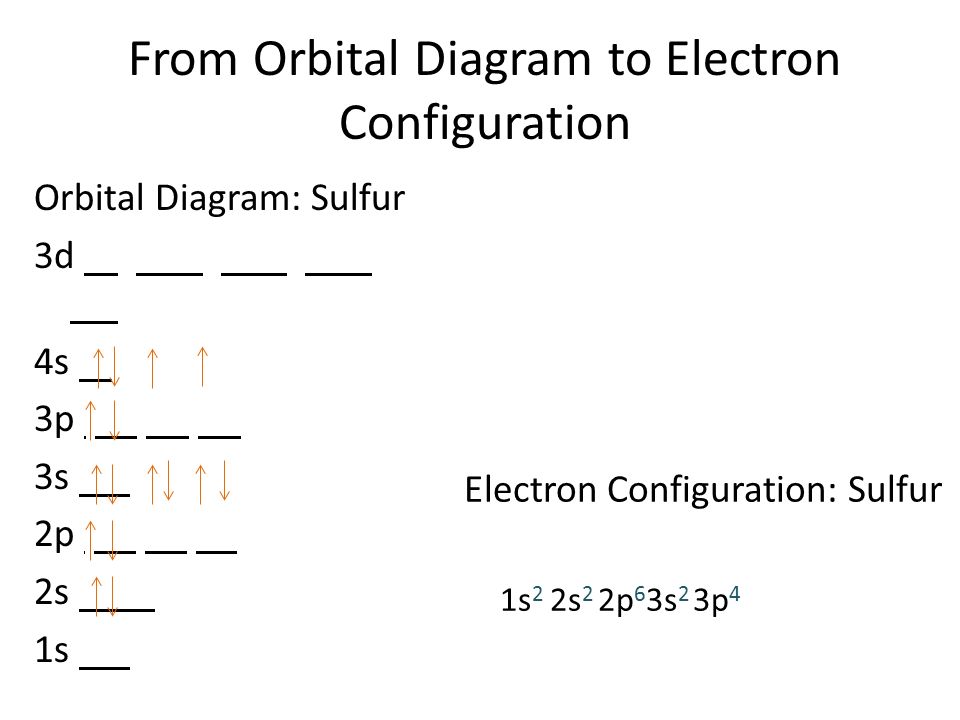
Sulfur Electron Configuration (S) with Orbital Diagram
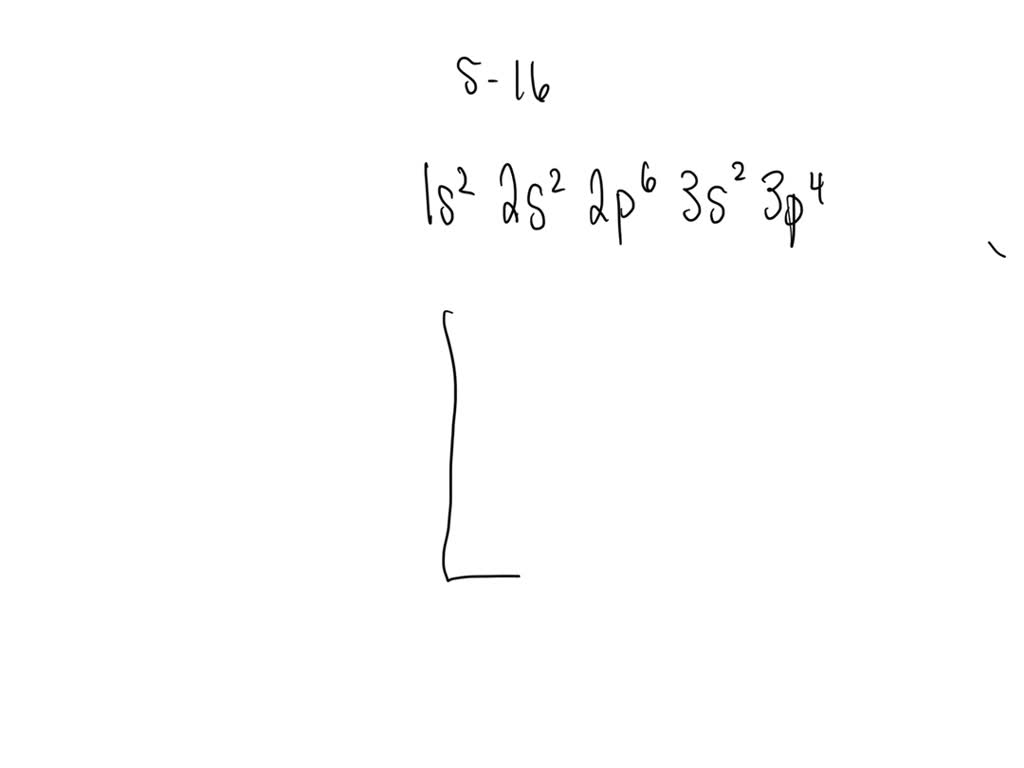
SOLVED Draw the electron configuration for a neutral atom of sulfur.

Diagram representation of the element sulfur Vector Image
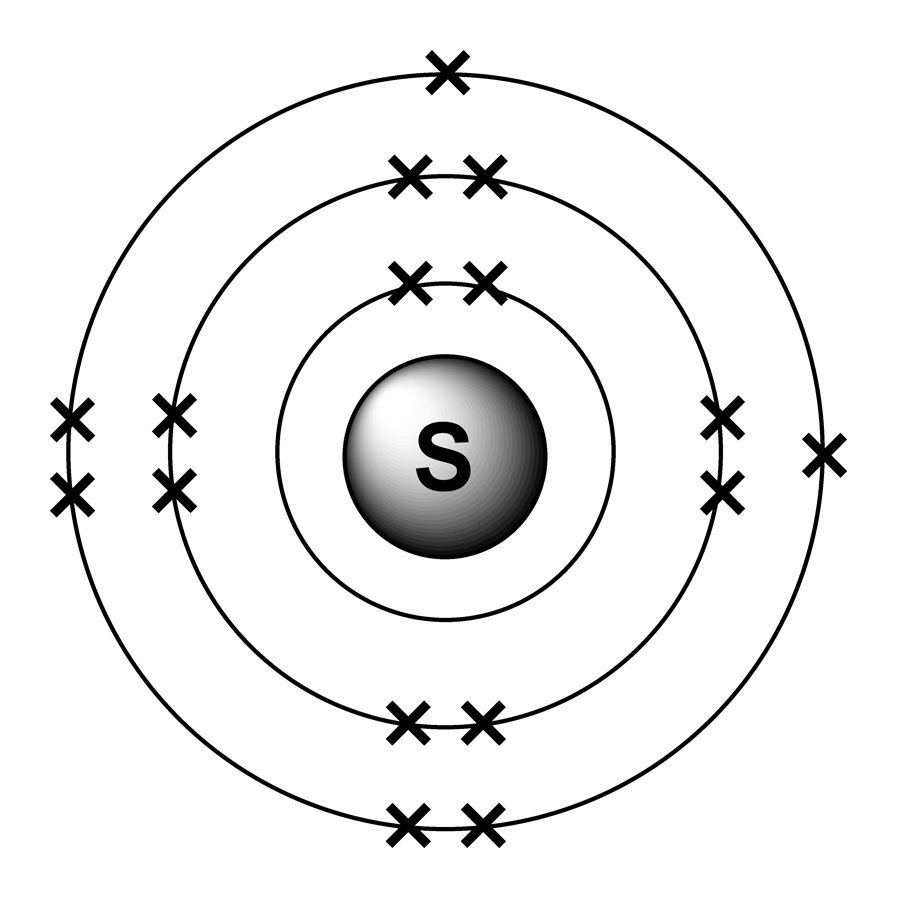
Sulfur Table of Elements by Shrenil Sharma
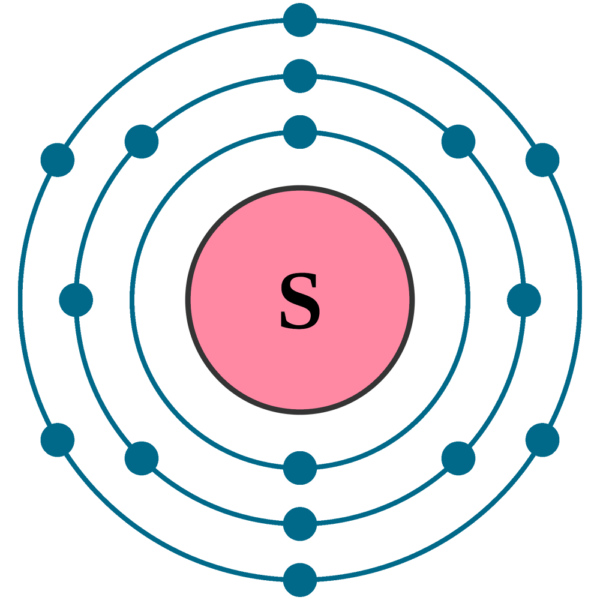
Sulfur S (Element 16) of Periodic Table Elements FlashCards

Sulfur Atom Science Notes and Projects
SOLVED Draw the electron configuration for a neutral atom of sulfur.

Draw The Electron Configuration For A Neutral Atom, HD Png Download
:max_bytes(150000):strip_icc()/sulfuratom-58b602563df78cdcd83d5a9d.jpg)
Atom Diagrams Electron Configurations of the Elements
The Aufbau Principle States That Electrons Are Always Placed In The.
Web For Hydrogen, Therefore, The Single Electron Is Placed In The 1S Orbital, Which Is The Orbital Lowest In Energy (Figure \(\Pageindex{1}\)), And The Electron Configuration Is Written As.
In Order To Maximize Their Stability, Electrons Will Always Prefer To Occupy The Lowest Energy Orbital.
When Applying This Pattern, Two Rules Must Be Followed:
Related Post: