Draw All Resonance Structures For The Sulfur Trioxide Molecule So3
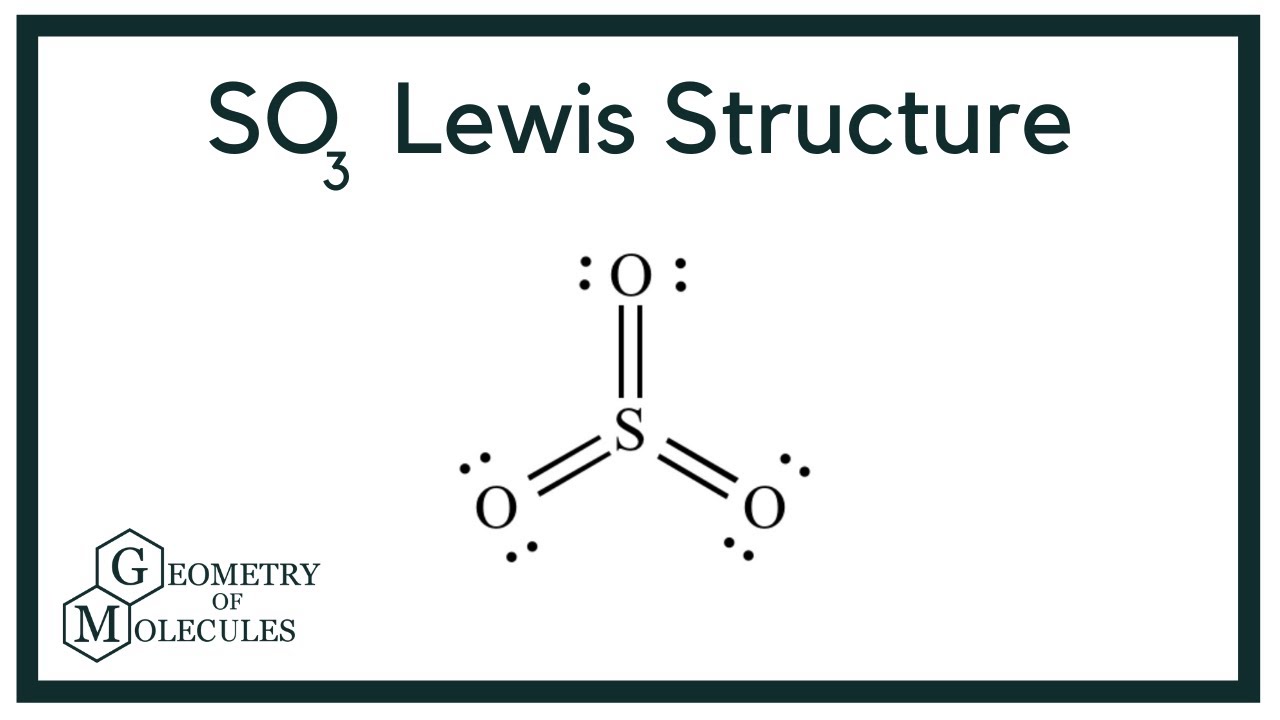
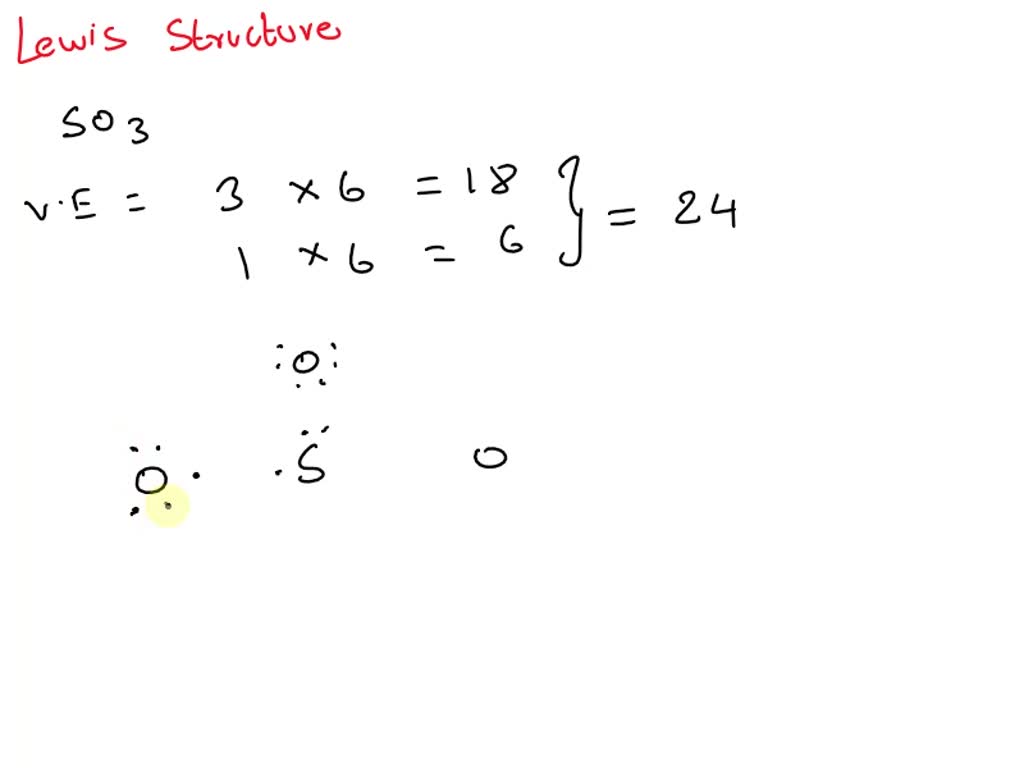
Draw All Resonance Structures For The Sulfur Trioxide Molecule So3 - Do not show ion charges in your drawings. Molecular formula and molecular geometry. > when you draw the lewis structure, you first get the three structures at the top. The three oxygens are drawn in the shape of a triangle with the nitrogen at the center of the triangle. Draw a structure for benzene. Find the total valence electrons in so3 molecule. It is determined with the help of formula: It will hold more than 8 electrons. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. But, there is no lone pair on sulfur atom in so 3 lewis structure as lewis structure of so 2. Lewis dot of sulfur trioxide. Web the so 3 lewis structure illustrates how the atoms of sulfur trioxide, a compound composed of one sulfur atom and three oxygen atoms, are arranged. Find the total valence electrons in so3 molecule. (valence electrons are the electrons that are present in. Use resonance structures to describe the bonding in benzene. > when you draw the lewis structure, you first get the three structures at the top. Note that so3 is a bi. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. Web steps of drawing so3 lewis structure step 1: The three oxygens are drawn. It is a form of pollution. Web lewis structure of so 3 molecule. Draw the lewis structure for sulfur trioxide following the octet rule. Sulfur having valence electrons in the 3rd energy level, would also have access to the 3d sublevel, thus allowing for more than 8 electrons. Web there are three resonance structures so3 (sulfur trioxide). Web this revised lewis structure, with double bonds between sulfur and oxygen atoms, is the best representation of so3, sulfur trioxide. In the molecule, there are a total of valence electrons. > when you draw the lewis structure, you first get the three structures at the top. Each oxygen atom has two lone pairs in so 3 lewis structure. Note. Add octet electrons to the atoms bonded to the center atom: There are 2 steps to solve this one. Within the so 3 lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Molecular formula and molecular geometry. (1*5) + (3*6) + 1 (ion) = 24 electrons. S does not follow the octet rule. Note that so3 is a bi. Web steps of drawing so3 lewis structure step 1: Web the benzene molecule (\(\ce{c6h6}\)) consists of a regular hexagon of carbon atoms, each of which is also bonded to a hydrogen atom. It discusses the molecular geometry, bond angle, hybridization, and. Lewis dot of sulfur trioxide. In each of the three structures in the middle, s has a formal charge of. Web there are three resonance structures so3 (sulfur trioxide). Each oxygen atom has two lone pairs in so 3 lewis structure. Number of hybrid orbitals = number of sigma bonds + number of lone pairs. Use resonance structures to describe the bonding in benzene. In the lewis structure, there are single bonds, double bonds, and. It discusses the molecular geometry, bond angle, hybridization, and. Lewis dot of sulfur trioxide. > when you draw the lewis structure, you first get the three structures at the top. Draw a structure for benzene. It is a form of pollution. S does not follow the octet rule. In each of the three structures in the middle, s has a formal charge of. So 3 is named sulfur trioxide. 70 more lewis dot structures. So 3 is named sulfur trioxide. It will hold more than 8 electrons. There are three double bonds around sulfur atom with oxygen atoms in so molecule. We start with a valid lewis structure and then follow these general rules. There are 2 steps to solve this one. Use resonance structures to describe the bonding in benzene. Web this problem has been solved! Molecular formula and molecular geometry. Do not show ion charges in your drawings. Draw one structure per sketcher box. We start with a valid lewis structure and then follow these general rules. Hybridization of so 3 molecule. Do not include overall ion charges or formal charges in your drawing. (valence electrons are the electrons that are present in. It will hold more than 8 electrons. Be sure to include all resonance structures that satisfy the octet rule: So 3 is named sulfur trioxide. There are seven resonance structures for so_3. In the molecule, there are a total of valence electrons. 70 more lewis dot structures.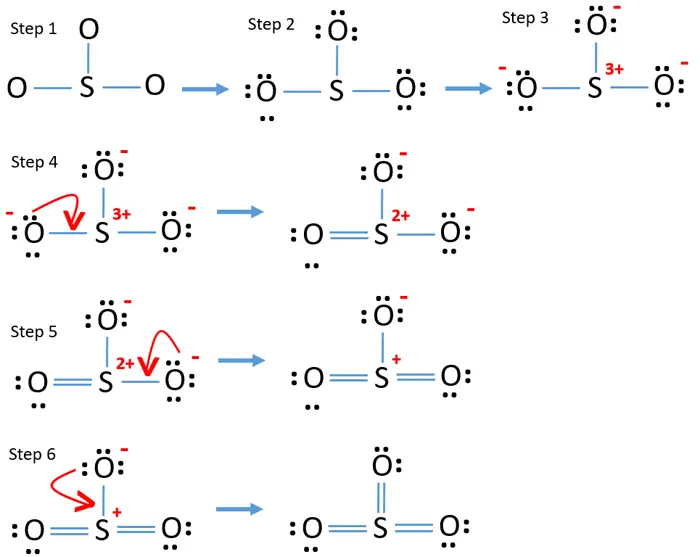
steps of drawing SO3 lewis structure VSEPR method
SO3 Lewis Structure (Sulfur Trioxide) YouTube
SOLVED Draw the Lewis structure for the sulfur trioxide 'SO3 molecule

SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube

SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur

SO3 Lewis Structure, Molecular Geometry, and Hybridization

Resonance Structures for SO3 (Sulfur trioxide) YouTube
Lewis Structures of Sulfur Trioxide (SO3) & Electrostatic Potentials

SO3 Lewis StructureLewis structure of SO3 (Sulfur trioxide) YouTube
By Following These Steps, You Can Accurately Draw The Lewis Structure For So3, Ensuring That All Atoms Meet The Octet Rule Or Expanded Octet Rule Where Needed And That The Molecule’s Formal Charges Are Balanced.
Web There Are Three Resonance Structures So3 (Sulfur Trioxide).
Web Lewis Structure Of So 3 Molecule.
Each Oxygen Atom Has Two Lone Pairs Of Electrons Associated With It.
Related Post: