Draw A Cell In A Hypertonic Solution
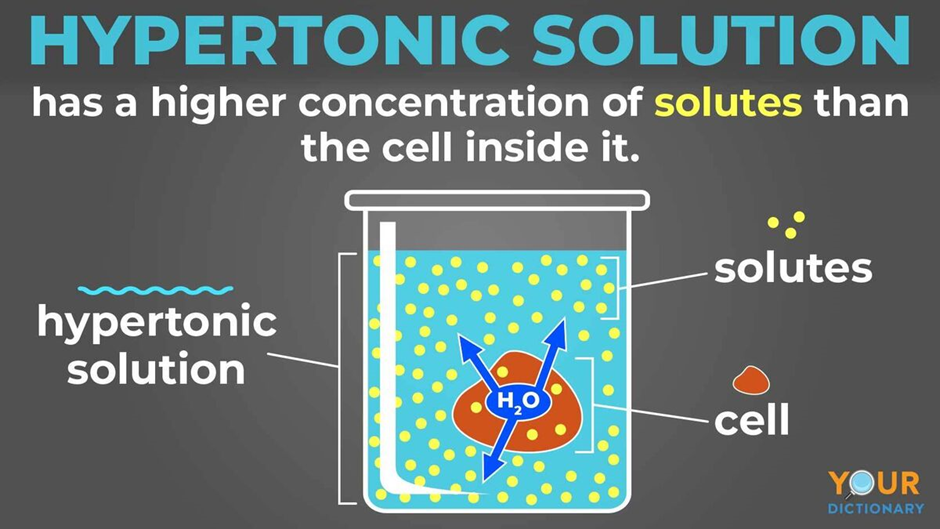
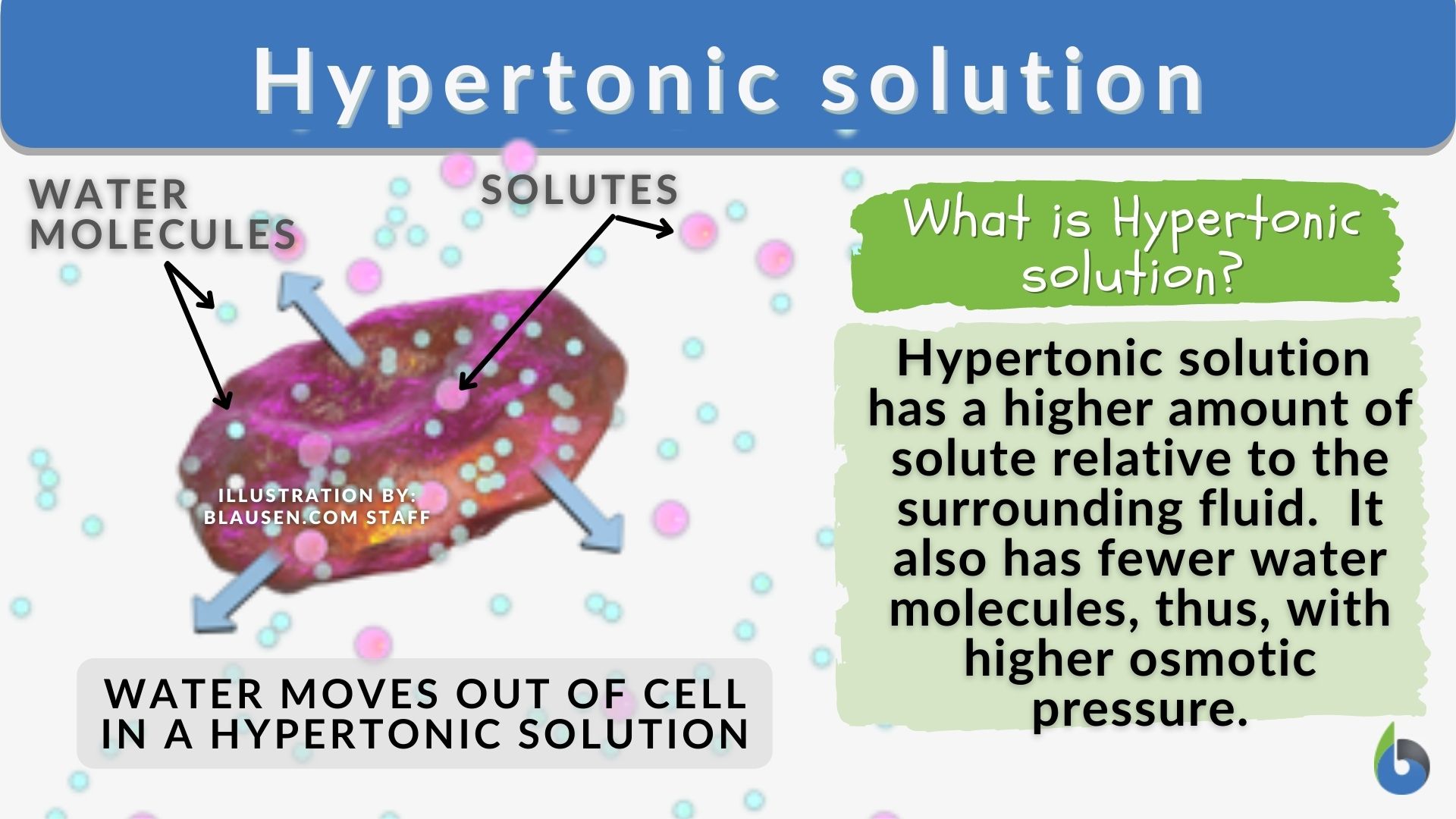
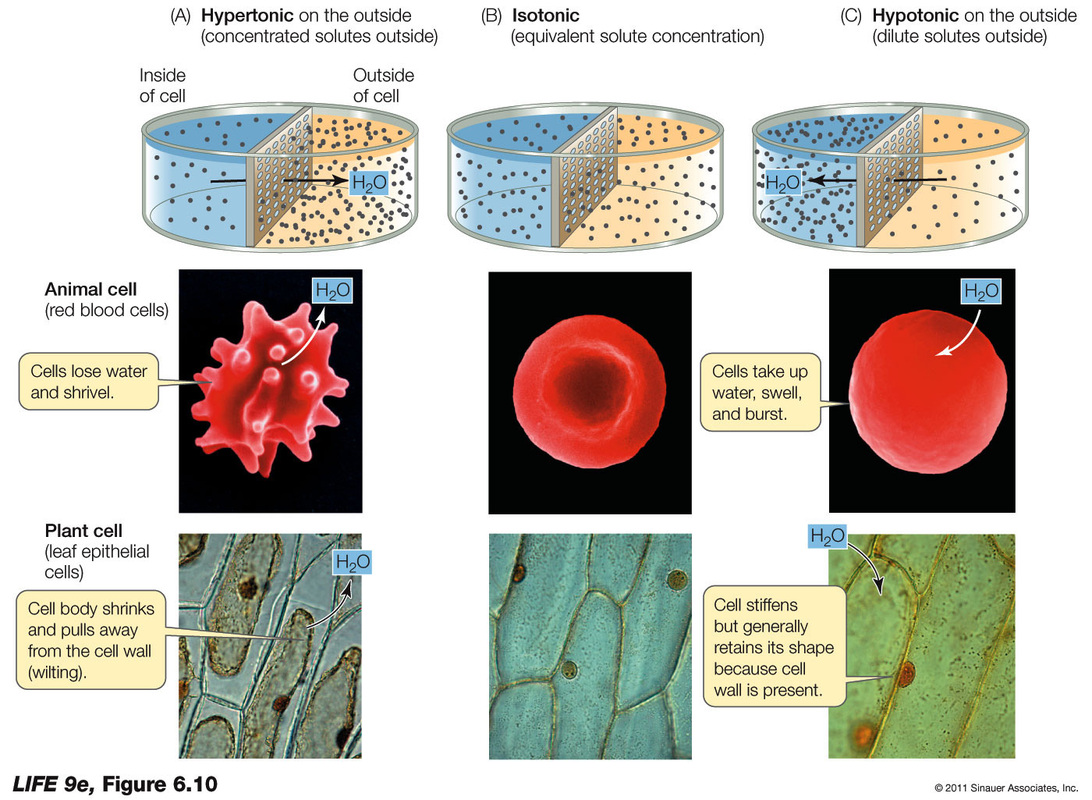
Draw A Cell In A Hypertonic Solution - These reactions are due to the semipermeable nature of cell membranes and. Key points osmolarity describes the total solute concentration of a solution; • a hypertonic solution has a higher solute (therefore, lower free water) concentration than the cell. In an isotonic solution, no net movement of water will take place. Web hypertonic solutions cause water molecules to move out of the cell and into the region of higher solute concentration. Web if a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until both solutions are isotonic. A hypertonic solution contains a high solute concentration with respect to cells. Hyper is a latin prefix meaning over or above. The plasma membrane pulls away from the cell wall as it shrivels, a process called plasmolysis. In this case, you can imagine that the solution is less concentrated than the cell’s cytoplasm, causing water from the solution to flow into the cell. Web in an isotonic solution, there is no net flow of water, keeping the cell stable. Hypertonic solutions have a higher solute concentration. Web what happens to a cell in a hypertonic solution? Remember, water moves from a region of low osmolarity to a region of high osmolarity. A hypertonic solution contains a high solute concentration with respect to cells. Web when cells are placed in an isotonic solution, there is no net movement of water. Web cells in a hypertonic solution shrink as water exits the cell, becoming shriveled. The cell would then expand and eventually lyse or burst. In a hypertonic solution, water leaves the cell, causing it to shrivel. Web study with quizlet and memorize flashcards containing. Web in a hypertonic environment, cells use membrane proteins called aquaporin channels to take advantage of the osmotic pressure gradient and shift water towards the higher concentration medium. Web an isotonic solution (for example, the ecf) has the same osmotic pressure as the icf. Hyper is a latin prefix meaning over or above. Marie look | feb 7, 2024. Remember,. In this case, since the extracellular fluid has low osmolarity, the water would rush into the cell. Web a solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. This causes water to rush out making the cell wrinkle or shrivel. This process is critical. Web study with quizlet and memorize flashcards containing terms like draw a labelled diagram that shows the positions of proteins within the cell membrane. In a hypertonic solution, water leaves the cell, causing it to shrivel. Web a cell placed into a hypertonic solution will shrivel and die by a process known as plasmolysis. Solutions with a low solute concentration. Web hypertonic solutions cause water molecules to move out of the cell and into the region of higher solute concentration. • a hypertonic solution has a higher solute (therefore, lower free water) concentration than the cell. This is clearly seen in red blood cells undergoing a process called crenation. For example, a solution containing 10% salt is hypertonic. Web if. If a solution is hypotonic to a cell, then the cell will swell when placed in the hypotonic solution. When plant cells are placed in such solutions, water will move from inside the plant cell to the outside of the cell, resulting in the shrinking of the cell (the cell is said to be plasmolyzed). Web hypertonic solutions have a. Web in a hypertonic solution, a cell with a cell wall will lose water too. Conversely, in hypotonicsolutions there is a higher solute concentration inside the cell than outside, and. Web a solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. Animal cells tend. Animal cells tend to do best in an isotonic environment, plant cells tend to do best in a hypotonic environment. Web what happens to a cell in a hypertonic solution? Hypertonic solutions have a higher solute concentration. Web a cell placed into a hypertonic solution will shrivel and die by a process known as plasmolysis. Web in a hypertonic solution,. A hypertonic solution contains a high solute concentration with respect to cells. Web what happens to a cell in a hypertonic solution? A hypertonic solution is one which has a higher solute concentration than another solution. Web if a cell is placed in a hypertonic solution, the cell is considered hypotonic. Web in an isotonic solution, there is no net. (4 marks), draw a labelled diagram of a eukaryotic plant cell as seen in an electron micrograph (4 marks) and more. Cells are permeable to water, and thanks to this, they can shrink and elevate the concentration of intracellular solutes. A hypertonic solution contains a higher concentration of solutes compared to another solution. A hypertonic solution is one which has a higher solute concentration than another solution. An isotonic solution is any external solution that has the same solute concentration and water concentration compared to body fluids. This process is critical to understand, especially in fields like biology, microbiology, and medicine, as it affects cellular function and health. Web if a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until both solutions are isotonic. Hyper is a latin prefix meaning over or above. Therefore, a hypertonic solution has more solutes than the intracellular environment, so water will leave the cell to try to achieve equilibrium. Web if a cell is placed in a hypertonic solution, the cell is considered hypotonic. Web if a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until both solutions are isotonic. These reactions are due to the semipermeable nature of cell membranes and. Cells placed in a hypotonic solution will take in water across their membranes until both the external solution and the cytosol are isotonic. The plasma membrane pulls away from the cell wall as it shrivels, a process called plasmolysis. • a hypertonic solution has a higher solute (therefore, lower free water) concentration than the cell. Web in a hypertonic environment, cells use membrane proteins called aquaporin channels to take advantage of the osmotic pressure gradient and shift water towards the higher concentration medium.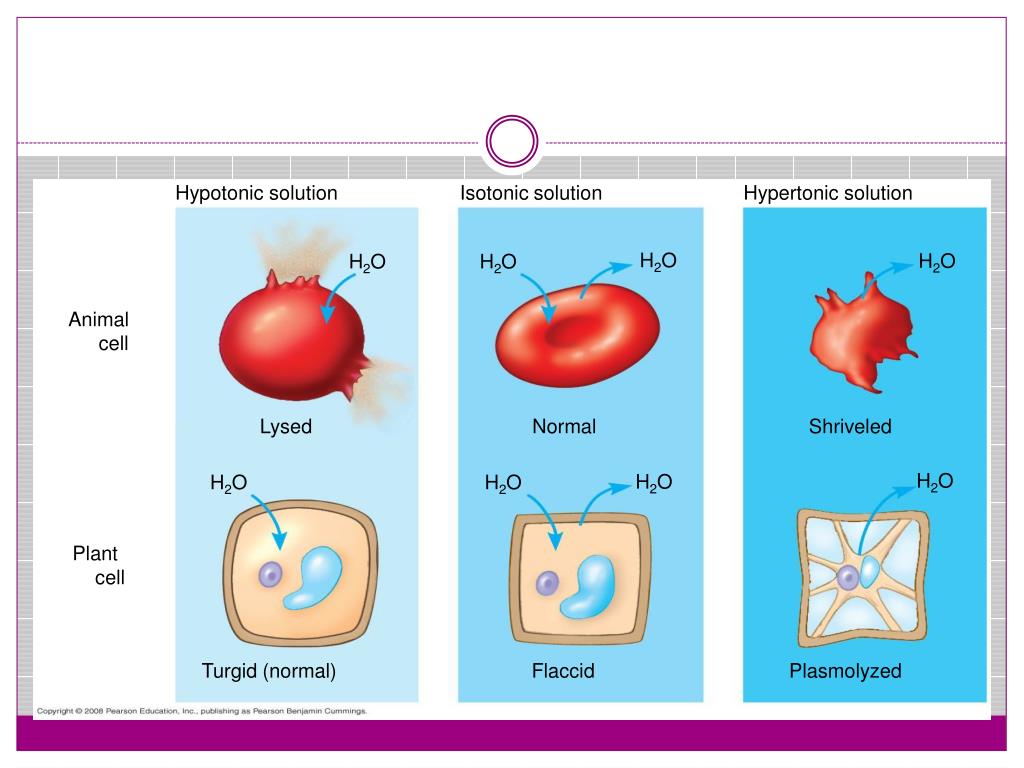
Animal Cell Vs Plant Cell In Hypertonic Solution Sanimale Gambaran

ITS WORTH 150 POINTS Draw a cell in a hypertonic solution. Make sure to
Hypertonic solution Definition and Examples Biology Online Dictionary

PPT CHAPTER 7 CELL STRUCTURE AND FUNCTION PowerPoint Presentation
Hypertonic solution Definition and Examples Biology Online Dictionary
Animal Cell In Hypertonic Solution / SPM Biology Types of Solution

Types of solutionshypertonic hypotonic and isotonic explained YouTube
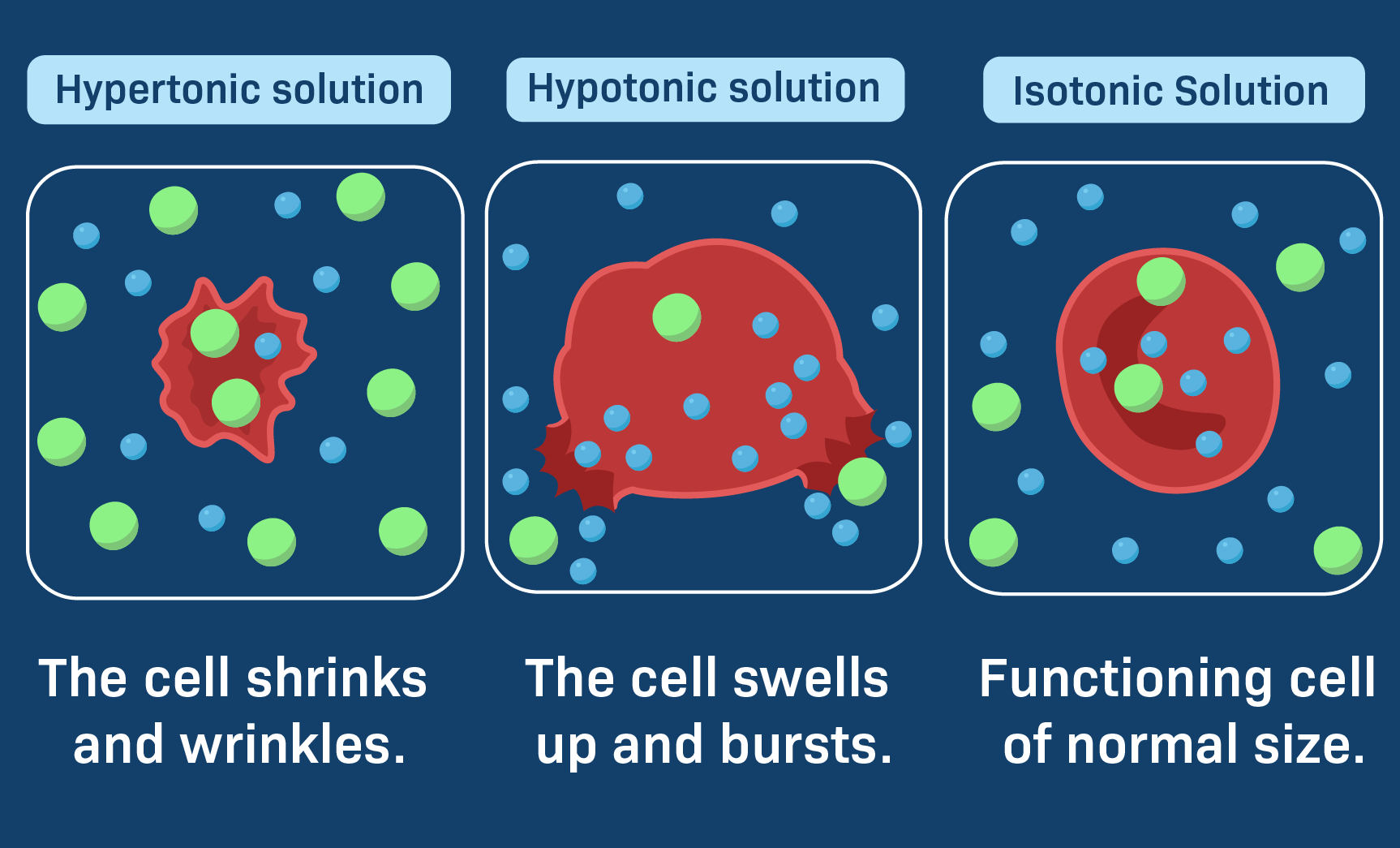
LabXchange

Plant Cell in Hypertonic Solution

Plant Cell in Hypertonic Solution
Web An Isotonic Solution (For Example, The Ecf) Has The Same Osmotic Pressure As The Icf.
In This Case, You Can Imagine That The Solution Is Less Concentrated Than The Cell’s Cytoplasm, Causing Water From The Solution To Flow Into The Cell.
Web Hypertonic Solutions Cause Water Molecules To Move Out Of The Cell And Into The Region Of Higher Solute Concentration.
Plant Cells In A Hypertonic Solution Can Look Like A Pincushion Because Of What’s Going On Inside.
Related Post: