Covid Test Template
Covid Test Template - Edit your negative covid test template online. Please refer to the template for test developers of molecular and antigen. This doc template contains all the. Web all materials are free for download. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. You can upload your logo, change fonts and colors, personalize text, add. Type text, add images, blackout confidential details, add comments, highlights and more. Sign it in a few clicks. They may be printed on a standard office printer, or you may use a commercial printer. National center for immunization and. You can upload your logo, change fonts and colors, personalize text, add. Sign it in a few clicks. Please refer to the template for test developers of molecular and antigen. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. Web all materials. This doc template contains all the. Hhs and cdc’s new guidance [288 kb, 9 pages] no longer requires reporting of. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. They may be printed on a standard office printer, or you may use. You can upload your logo, change fonts and colors, personalize text, add. The fda has posted a new template for commercial developers to submit emergency use authorization (eua) requests for. If you have questions or would like to discuss. Edit your negative covid test template online. Negative test declaration form, serves a documentation that you have undergone a viral test. You can upload your logo, change fonts and colors, personalize text, add. This doc template contains all the. National center for immunization and. Type text, add images, blackout confidential details, add comments, highlights and more. The fda has posted a new template for commercial developers to submit emergency use authorization (eua) requests for. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. You can upload your logo, change. Sign it in a few clicks. National center for immunization and. Please refer to the template for test developers of molecular and antigen. They may be printed on a standard office printer, or you may use a commercial printer. Negative test declaration form, serves a documentation that you have undergone a viral test (e.g. The fda has posted a new template for commercial developers to submit emergency use authorization (eua) requests for. Type text, add images, blackout confidential details, add comments, highlights and more. Sign it in a few clicks. You can upload your logo, change fonts and colors, personalize text, add. Web all materials are free for download. Web all materials are free for download. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. Sign it in a few clicks. Negative test declaration form, serves a documentation that you have undergone a viral test (e.g. The fda has posted a. This doc template contains all the. National center for immunization and. Hhs and cdc’s new guidance [288 kb, 9 pages] no longer requires reporting of. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. Web all materials are free for download. National center for immunization and. This doc template contains all the. Web all materials are free for download. Updates as of april 4, 2022. The fda has posted a new template for commercial developers to submit emergency use authorization (eua) requests for. This doc template contains all the. Negative test declaration form, serves a documentation that you have undergone a viral test (e.g. They may be printed on a standard office printer, or you may use a commercial printer. Please refer to the template for test developers of molecular and antigen. Hhs and cdc’s new guidance [288 kb, 9 pages] no longer requires reporting of. Updates as of april 4, 2022. Sign it in a few clicks. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. National center for immunization and. If you have questions or would like to discuss. Web this template provides the food and drug administration’s (fda) current recommendations concerning what data and information should be submitted to fda in support of a pre. Type text, add images, blackout confidential details, add comments, highlights and more.
NHS Tells Hospitals To Test All InPatients For Covid19, Including

COVID19 form YWCA Northwestern IL
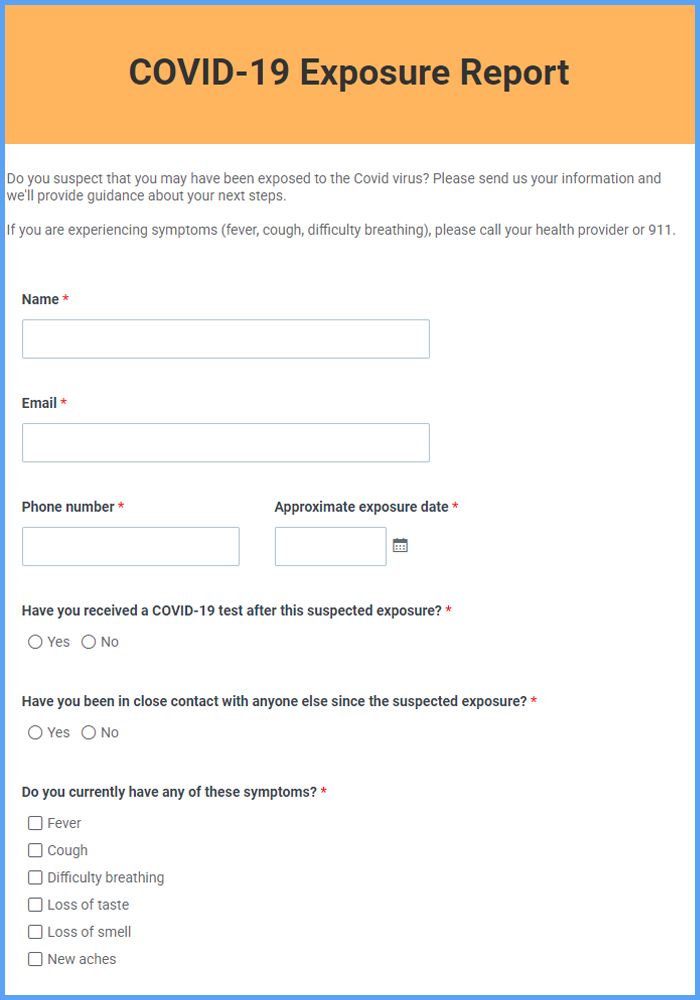
Covid Exposure Email Template

How to Print Your COVID19 Results Advanced Urgent Care & Occ Med

Positive covid test results template Fill out & sign online DocHub
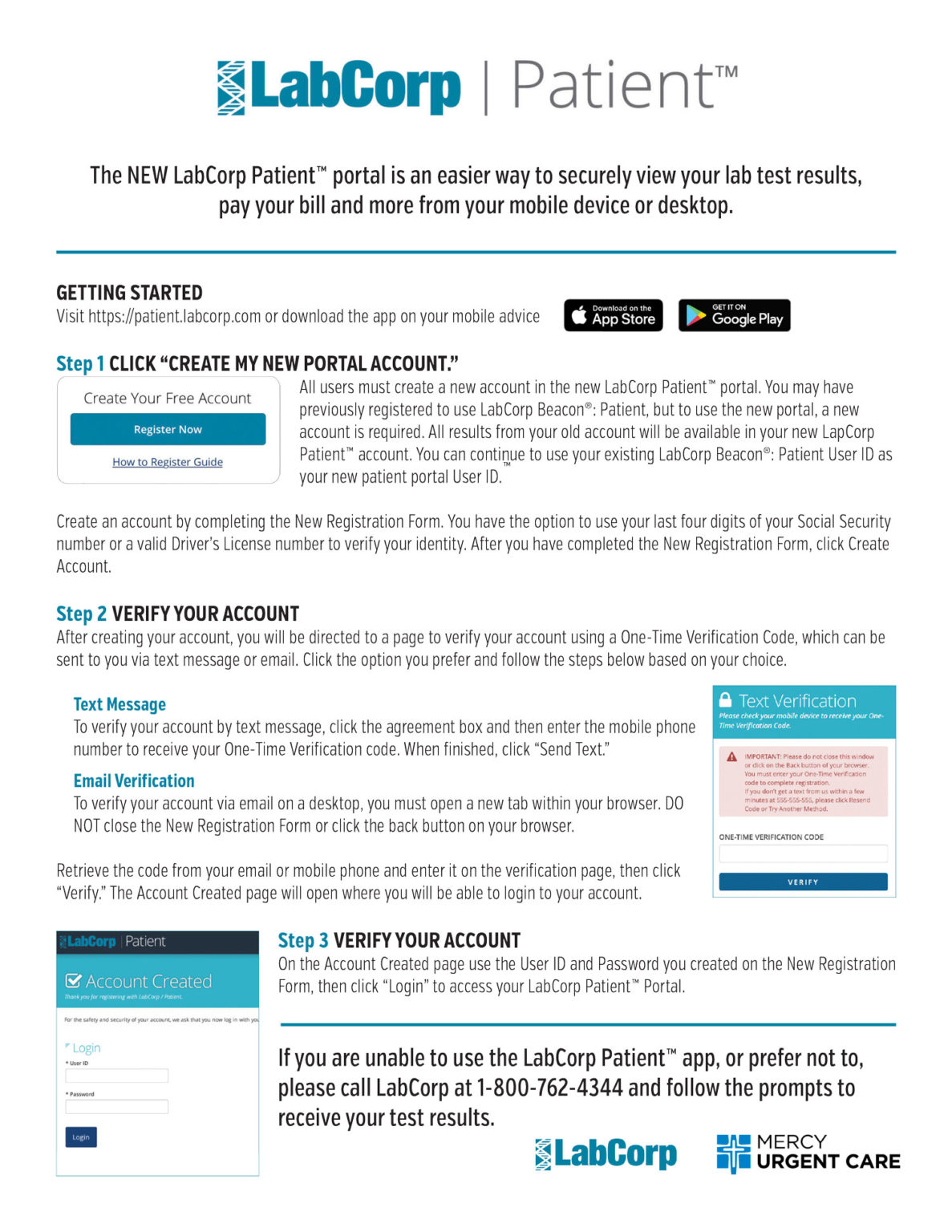
Get Your COVID19 Test Results Mercy Urgent Care
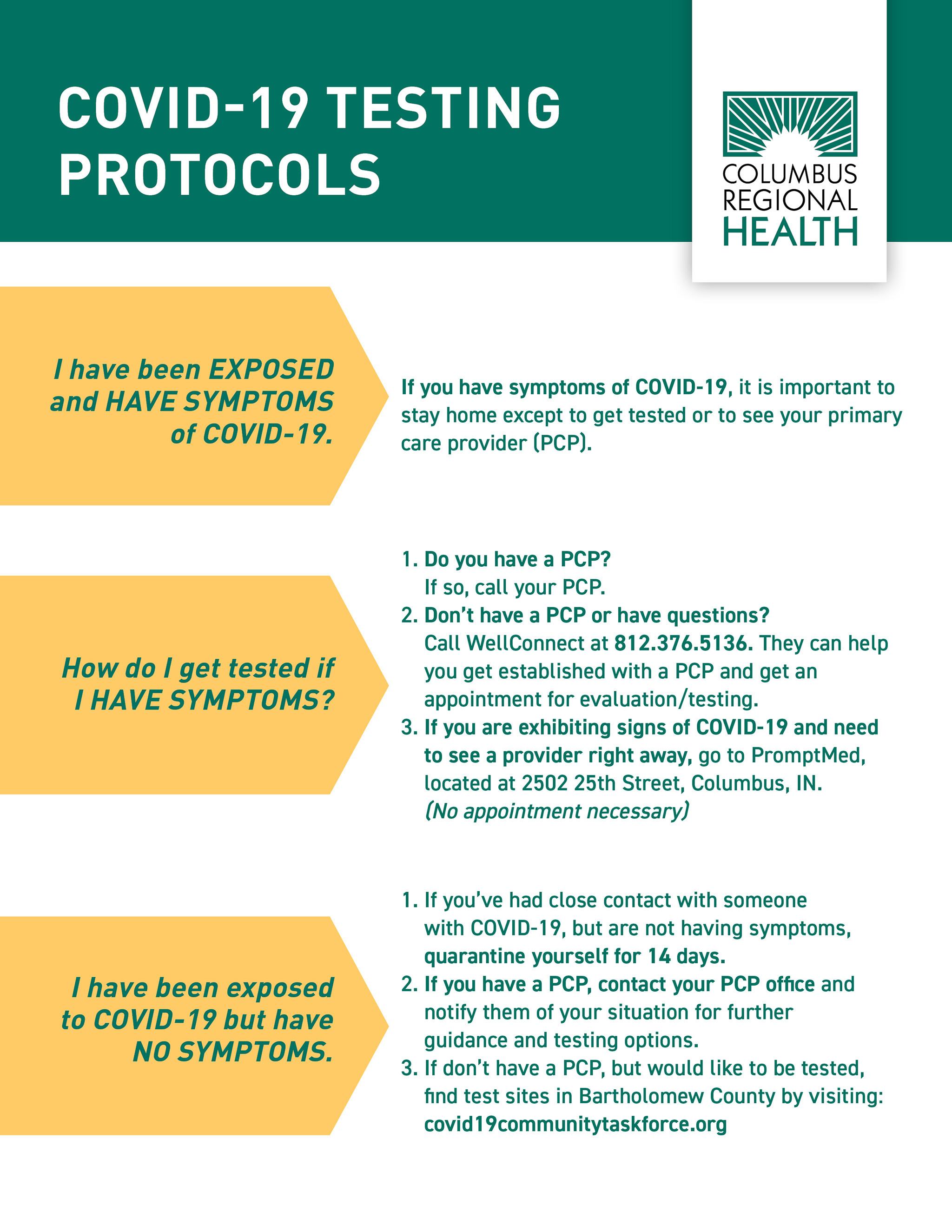
COVID19 Testing Protocols Columbus Regional Health

COVID19 ACM Claims

COVID19 screening questions used by federal corrections Canada.ca

Covid Rapid Test Template Complete with ease airSlate SignNow
Web All Materials Are Free For Download.
You Can Upload Your Logo, Change Fonts And Colors, Personalize Text, Add.
Edit Your Negative Covid Test Template Online.
The Fda Has Posted A New Template For Commercial Developers To Submit Emergency Use Authorization (Eua) Requests For.
Related Post: