Covalent Bond Drawing
Covalent Bond Drawing - Each h atom starts with a single electron in its valence shell: Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. Web a covalent bond is formed between two atoms by sharing electrons. These structural images are named after gilbert lewis, the american chemist who first proposed that covalent molecules could be represented visually. Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the 1s subshell. H forms only one bond because it needs only two electrons. This bonding allow atoms to have full outer shell of electrons. The video covers the basic lewis structures you'll see in an introductory chemistry class. Chemists frequently use lewis diagrams to represent covalent bonding in molecular substances. So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2). Web a covalent bond is formed between two atoms by sharing electrons. This bonding allow atoms to have full outer shell of electrons. Chemists frequently use lewis diagrams to represent covalent bonding in molecular substances. Web covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Web a covalent bond is formed between two atoms by sharing electrons. Web a discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of. Web a covalent bond is formed between two atoms by sharing electrons. H forms only one bond because it needs only two electrons. These structural images are named after gilbert lewis, the american chemist who first proposed that covalent molecules could be represented visually. The video covers the basic lewis structures you'll see in an introductory chemistry class. Web in. Each h atom starts with a single electron in its valence shell: This bonding allow atoms to have full outer shell of electrons. Only the electrons in the outer shell take part in the bonding. Web a discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of. So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2). Web when electrons are shared between two atoms, they form a covalent bond. Web a discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of. The video covers the basic lewis structures you'll see in an introductory chemistry class. Each h atom starts with a single electron in its valence shell: Let us illustrate a covalent bond by using h atoms, with the understanding that h atoms need only two electrons to fill the 1s subshell. Chemists frequently use lewis diagrams to represent covalent bonding. Each h atom starts with a single electron in its valence shell: H forms only one bond because it needs only two electrons. Web when electrons are shared between two atoms, they form a covalent bond. Chemists frequently use lewis diagrams to represent covalent bonding in molecular substances. These structural images are named after gilbert lewis, the american chemist who. Hydrogen is an exception to the octet rule. So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2). Web covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond. The video covers the basic lewis structures you'll see in an introductory chemistry class. H forms only one bond because it needs only two electrons. Chemists frequently use lewis diagrams to represent covalent bonding in molecular substances. Web covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half. H forms only one bond because it needs only two electrons. Chemists frequently use lewis diagrams to represent covalent bonding in molecular substances. Web a covalent bond is formed between two atoms by sharing electrons. Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. Hydrogen is an exception to the octet rule. Hydrogen is an exception to the octet rule. These structural images are named after gilbert lewis, the american chemist who first proposed that covalent molecules could be represented visually. H forms only one bond because it needs only two electrons. Web a discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. Web a covalent bond is formed between two atoms by sharing electrons. Web covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original valence electrons. The video covers the basic lewis structures you'll see in an introductory chemistry class. Only the electrons in the outer shell take part in the bonding. Web in this video you’ll learn how to draw lewis dot structures for covalent compounds. So yes each covalent bond will be a pair of electrons because each atom contributes 1 electron to a bond (and 1+1=2). Chemists frequently use lewis diagrams to represent covalent bonding in molecular substances. Web when electrons are shared between two atoms, they form a covalent bond.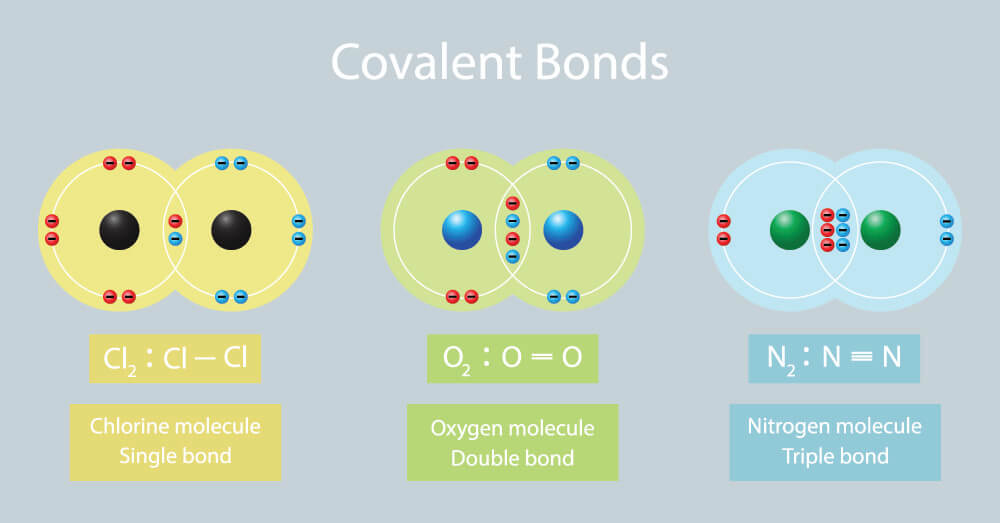
Covalent Bond Biology Dictionary

How is a covalent bond formed

Covalent Bonding The Science and Maths Zone

Covalent Bonding The Science and Maths Zone
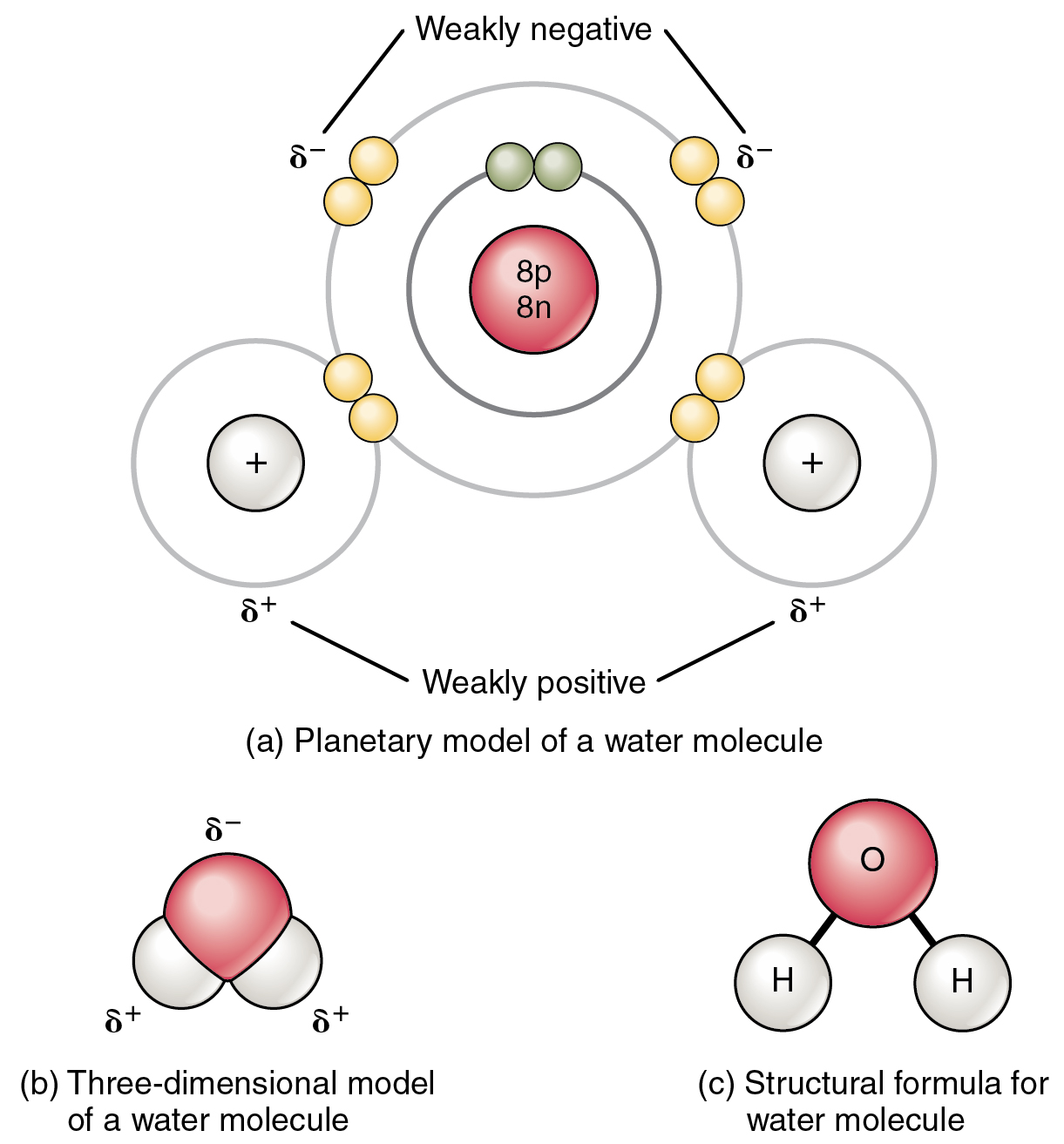
Chemical Bonds · Anatomy and Physiology
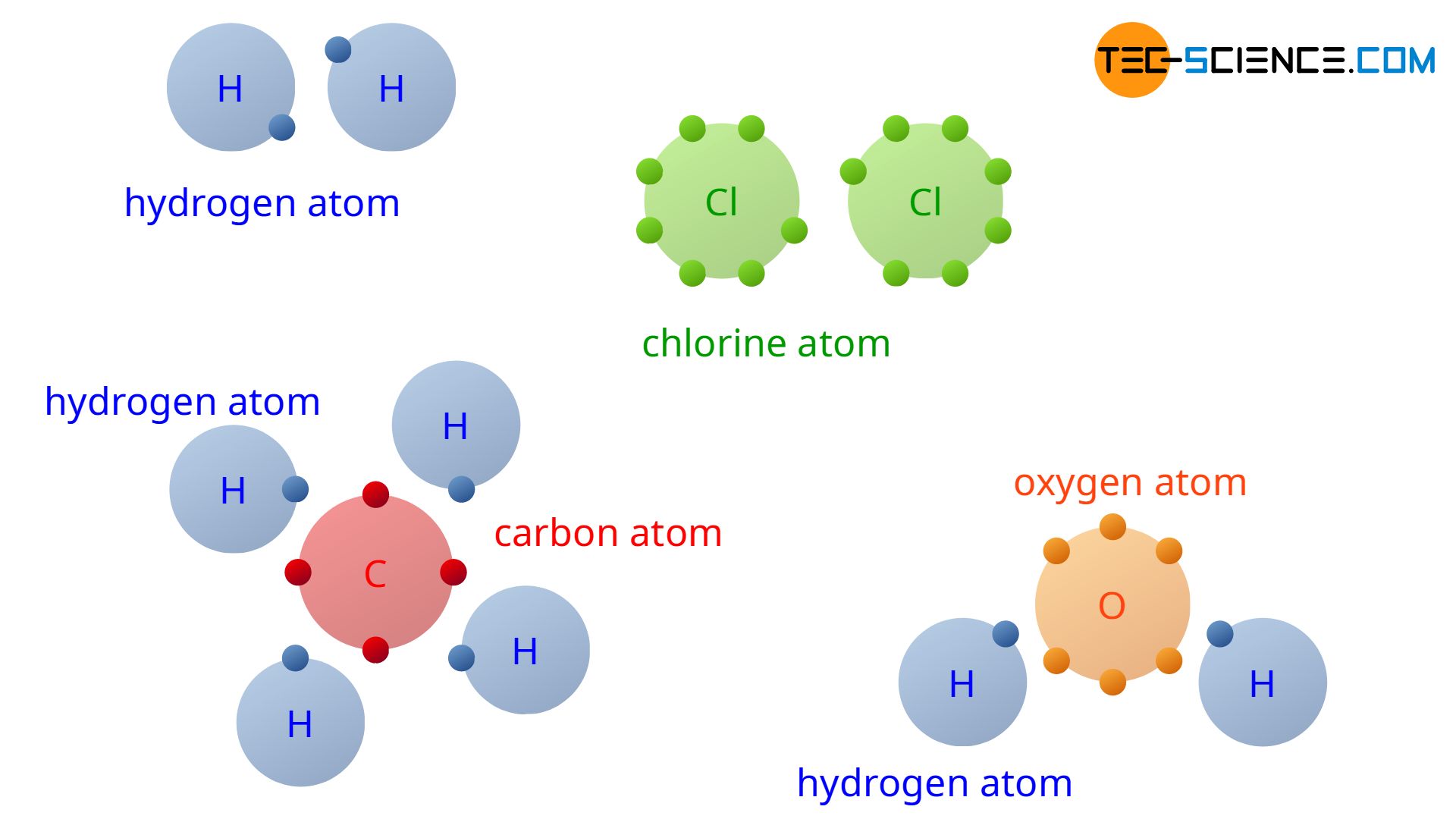
Covalent bonding tecscience
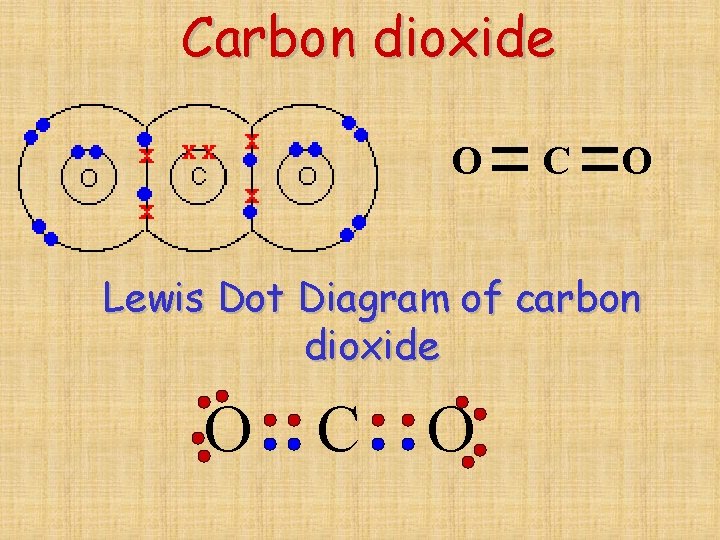
Chapter 8 Covalent Bonding Covalent bonding Usually forms
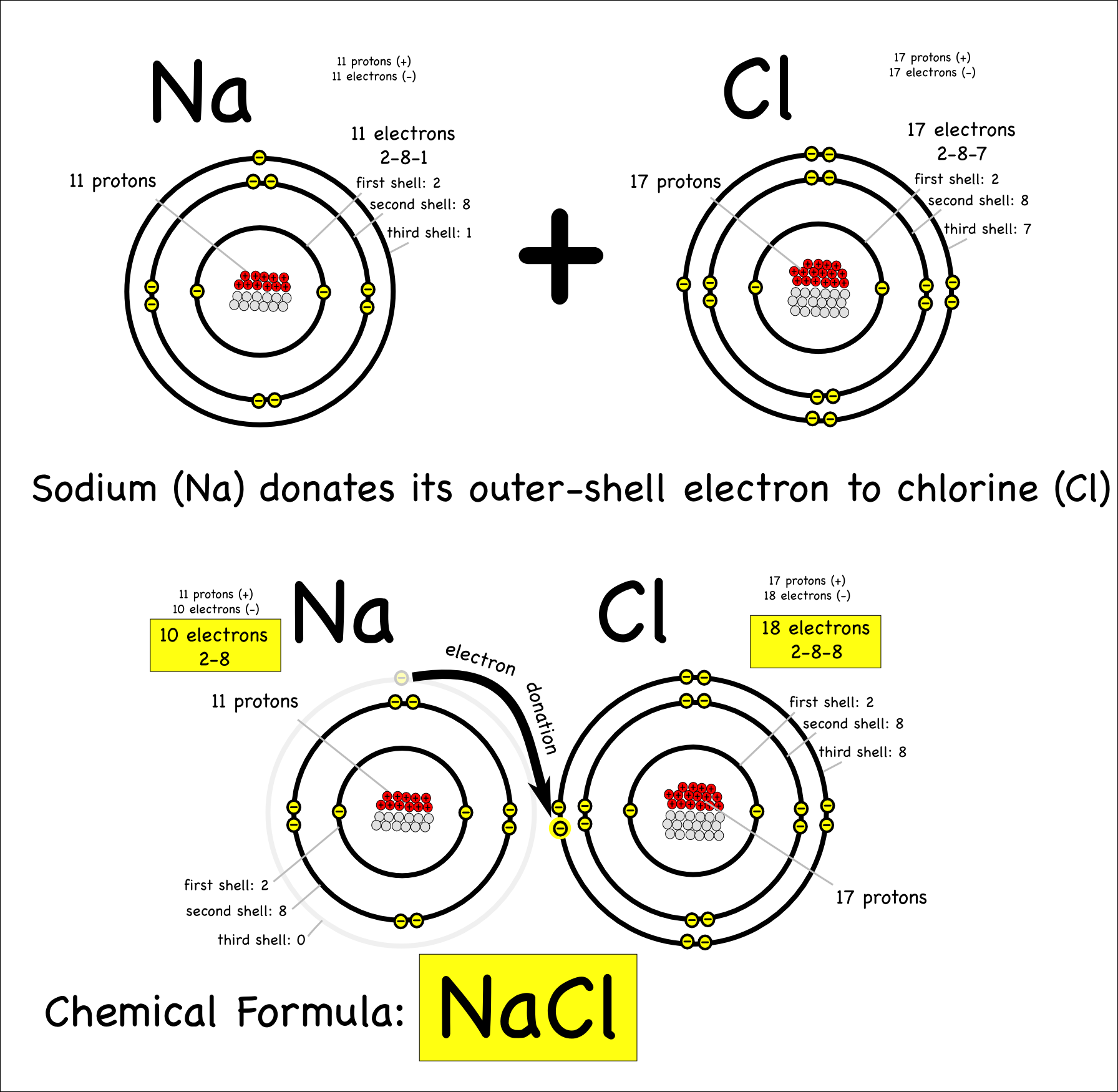
Introducing Covalent Bonding Montessori Muddle
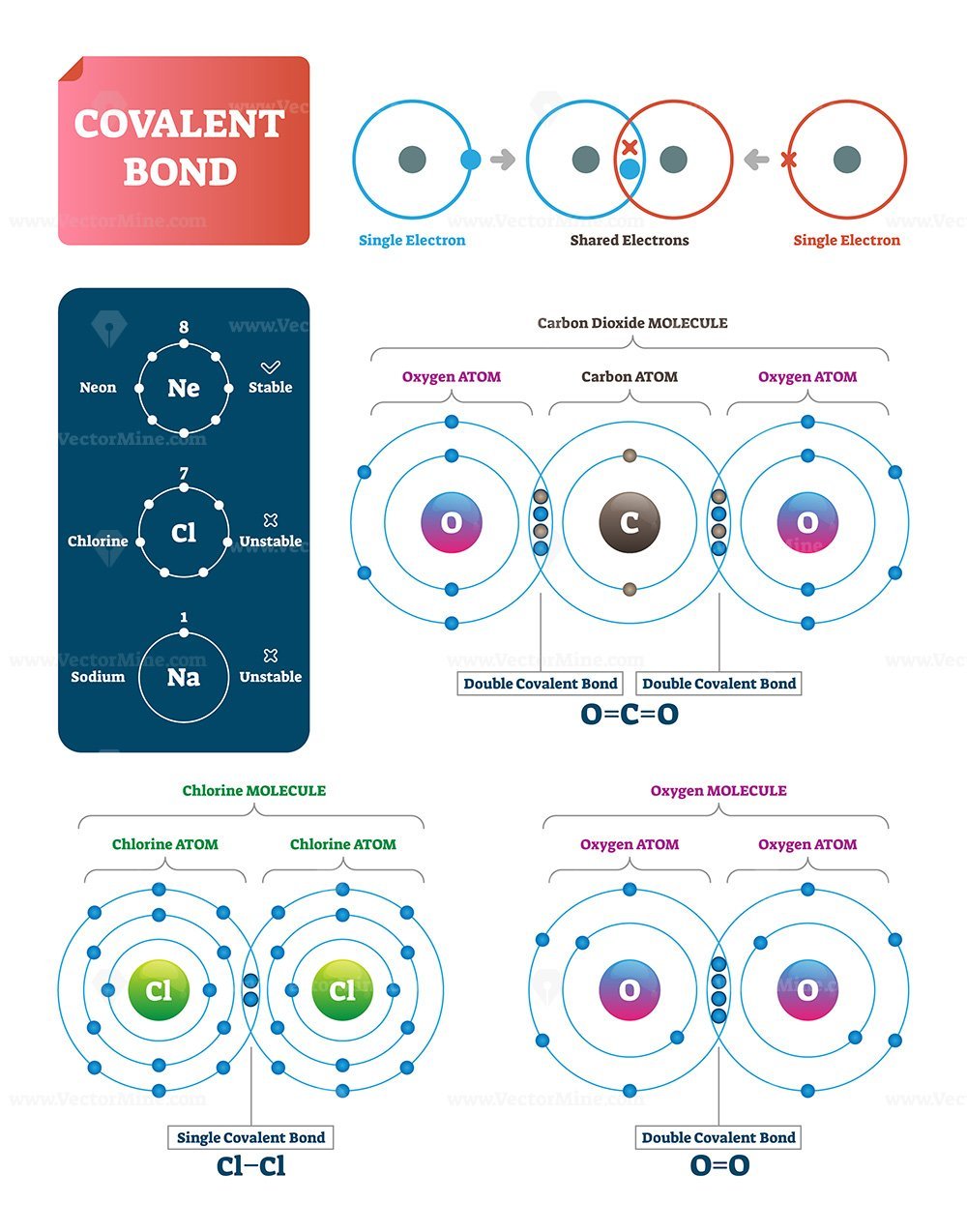
Covalent bond vector illustration VectorMine
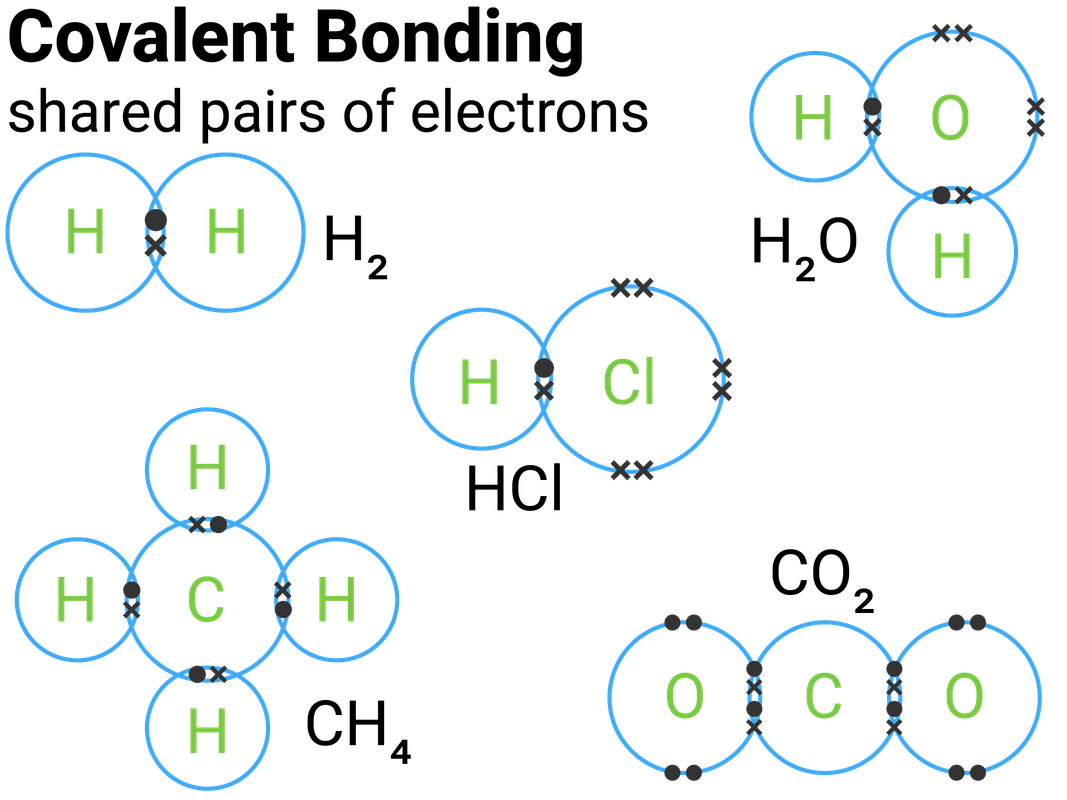
Ethene Covalent Bond Diagram
Let Us Illustrate A Covalent Bond By Using H Atoms, With The Understanding That H Atoms Need Only Two Electrons To Fill The 1S Subshell.
Each H Atom Starts With A Single Electron In Its Valence Shell:
This Bonding Allow Atoms To Have Full Outer Shell Of Electrons.
The Number Of Bonds An Element Forms In A Covalent Compound Is Determined By The Number Of Electrons It Needs To Reach Octet.
Related Post: