Consort Flow Diagram Template
Consort Flow Diagram Template - Assessed for eligibility (n= ) excluded (n= ) ♦. Information required to complete a consort flow diagram includes the. Add new column to attrition dataframe. Not meeting inclusion criteria (n= ) ♦. Web a consort diagram presents the flow of subjects at each stage in a clinical trial. = give reasons n =. Web the consort 2010 statement provides updated guidelines for reporting parallel group randomised trials, including the flow diagram template. Web the template for the consort flow diagram [1,2] is shown in figure figure1. The top three boxes in this example diagram displays patients count for screening, screen failure and randomization respectively. Analysed (n= ) excluded from analysis (give reasons). Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) Web consort 2010 flow diagram. Web the template for the consort flow diagram [1, 2] is shown in figure 1. Fill out & sign online | dochub. Web figure 1.sample consort diagram. Consort flow diagram of the progress through the phases of a parallel randomised trial of two groups (that is, enrolment, intervention allocation, follow. Web the template for the consort flow diagram [1,2] is shown in figure figure1. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a. Analysed n = excluded from analysis n = give reasons n =. Web consort flow diagram for crossover trials (would need modification if more than two groups and/or periods). Web consort 2010 flow diagram. Fill out & sign online | dochub. Assessed for eligibility (n= ) excluded (n= ) ♦. Analysed n = excluded from analysis n = give reasons n =. The flow diagram can be. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Web consort 2010 checklist of information to include when reporting a randomised trial *. Fill out & sign online | dochub. Web consort flow diagram for crossover trials (would need modification if more than two groups and/or periods). The flow diagram can be. Other reasons (n= ) analysed (n=. Information required to complete a consort flow diagram includes the. Web this article includes the 26 item checklist, a separate checklist for the abstract, a template for a consort flowchart for these studies, and an explanation of. Web consort diagram template ppt: Web the consort 2010 statement provides updated guidelines for reporting parallel group randomised trials, including the flow diagram template. Information required to complete a consort flow diagram includes. Web download a word and pdf file with a sample template for the consort diagram, a tool to describe the flow of participants through a randomized trial. Not meeting inclusion criteria (n= ) ♦. Add new column to attrition dataframe. Analysed (n= ) excluded from analysis (give reasons). The text boxes can be. Analysed (n= ) excluded from analysis (give reasons). Web the template for the consort flow diagram [1,2] is shown in figure figure1. Analysed n = excluded from analysis n = give reasons n =. Consort flow diagram of the progress through the phases of a parallel randomised trial of two groups (that is, enrolment, intervention allocation, follow. Declined to participate. = give reasons n =. Web this article includes the 26 item checklist, a separate checklist for the abstract, a template for a consort flowchart for these studies, and an explanation of. Web download a word and pdf file with a sample template for the consort diagram, a tool to describe the flow of participants through a randomized trial. The. Declined to participate (n= ) ♦. Assessed for eligibility (n= ) excluded (n= ) ♦. Not meeting inclusion criteria (n= ) ♦. Web a consort diagram presents the flow of subjects at each stage in a clinical trial. Web this article includes the 26 item checklist, a separate checklist for the abstract, a template for a consort flowchart for these. Fill out & sign online | dochub. Analysed (n= ) excluded from analysis (give reasons). Other reasons (n= ) analysed (n=. Web consort diagram template ppt: Web figure 1.sample consort diagram. Information required to complete a consort flow diagram includes the. Add new column to attrition dataframe. Web this article includes the 26 item checklist, a separate checklist for the abstract, a template for a consort flowchart for these studies, and an explanation of. = give reasons n =. The top three boxes in this example diagram displays patients count for screening, screen failure and randomization respectively. Not meeting inclusion criteria (n= ) ♦. Web the consort 2010 statement provides updated guidelines for reporting parallel group randomised trials, including the flow diagram template. Declined to participate (n= ) ♦. Information required to complete a consort flow diagram includes the number of. Web download a word and pdf file with a sample template for the consort diagram, a tool to describe the flow of participants through a randomized trial. The flow diagram can be.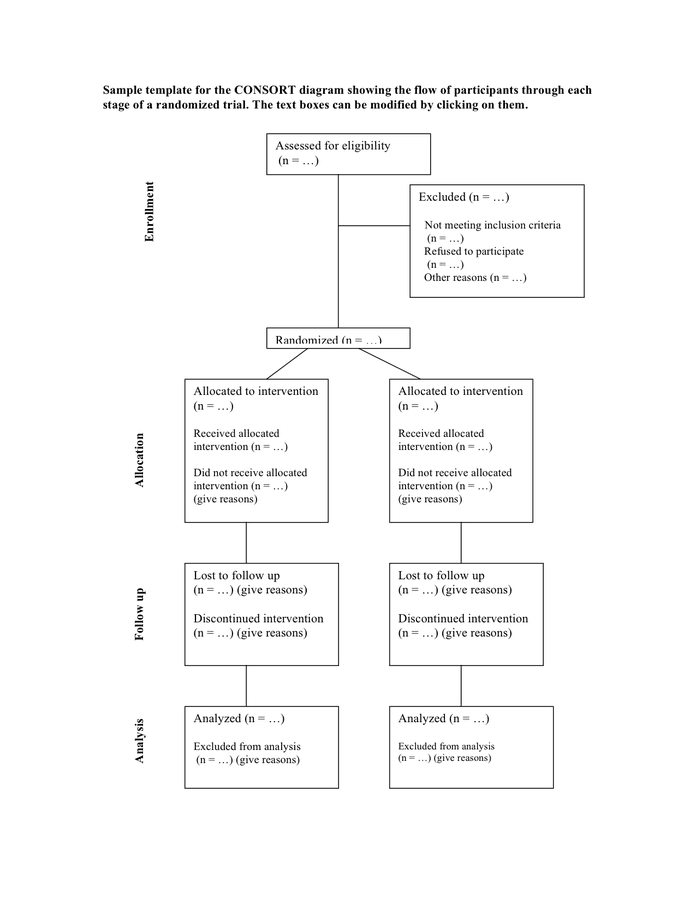
Consort Flow Diagram Template

Consort Flow Diagram Template

CONSORT Flow Diagram. Consort diagram of patients eligible, recruited

CONSORT 2010 flow diagram. Download Scientific Diagram

Revised template of the CONSORT diagram showing the flow of

Consort Flow Diagram Template

CONSORT 2010 flow diagram. CONSORT flow diagram template courtesy of

Consort Flow Diagram Template

Consort Flow Diagram Template

CONSORT flow diagram 16 . Download Scientific Diagram
Web Consort 2010 Checklist Of Information To Include When Reporting A Randomised Trial * Section/Topic Item No Checklist Item Reported On Page No Title And Abstract 1A.
Web A Consort Diagram Presents The Flow Of Subjects At Each Stage In A Clinical Trial.
Web The Consort Statement (Or Simply Consort) Comprises A Checklist Of Essential Items That Should Be Included In Reports Of Rcts And A Diagram For.
Analysed N = Excluded From Analysis N = Give Reasons N =.
Related Post: